Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall - PubMed (original) (raw)
. 2010 Dec 16;468(7326):921-6.
doi: 10.1038/nature09576. Epub 2010 Nov 10.
Affiliations
- PMID: 21068723
- PMCID: PMC3026603
- DOI: 10.1038/nature09576
Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall
Yang Xiang et al. Nature. 2010.
Abstract
Photoreceptors for visual perception, phototaxis or light avoidance are typically clustered in eyes or related structures such as the Bolwig organ of Drosophila larvae. Unexpectedly, we found that the class IV dendritic arborization neurons of Drosophila melanogaster larvae respond to ultraviolet, violet and blue light, and are major mediators of light avoidance, particularly at high intensities. These class IV dendritic arborization neurons, which are present in every body segment, have dendrites tiling the larval body wall nearly completely without redundancy. Dendritic illumination activates class IV dendritic arborization neurons. These novel photoreceptors use phototransduction machinery distinct from other photoreceptors in Drosophila and enable larvae to sense light exposure over their entire bodies and move out of danger.
Figures
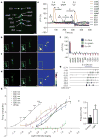
Figure 1. Photoreceptors in addition to Bolwig organs contribute to photoavoidance
a, b, Examples of light avoidance of wild-type (a) and GMR-Hid (b) larvae exposed to white light (0.57 mW mm−2) applied from 0 to 5 s. The light spot is indicated by the dotted circle. The arrow indicates the direction of larval locomotion; arrowheads at 2 s (a) and 5 s (b) indicate larval head turning. c–h, Percentage of animals avoiding white light (c), light of 360 nm (ultraviolet; d), 402 nm (violet; e), 470 nm (blue; f), 525 nm (green; g) and 620 nm (red; h) at different intensities. *P <0.05, **P <0.01, ***P <0.001, two-tailed Fisher exact test. Twenty to forty larvae were tested for each condition. Scale bar: 1 mm (a, b), shown at −2 s.
Figure 2. Light activates class IV dendritic arborization neurons
a, Pre-stimulation image showing larval dorsal cluster sensory neurons (dbd, bipolar dendrite neuron; ddaD and ddaE, class I dendritic arborization neurons; ddaB, class II dendritic arborization neurons; ddaA and ddaF, class III dendritic arborization neurons; ddaC, class IV dendritic arborization neurons; ES, external sensory organ). Up is dorsal; right is anterior. For an atlas of the larval peripheral nervous system, see ref. . b, Responses of the dorsal cluster neurons in a to 5 s blue light (470 nm) illumination. The boxed area in the left panel and insets in all three panels show the somas of ddaC, ddaF and ddaD dendritic arborization neurons. Left, pre-stimulation; middle, post-stimulation; right, GCaMP3 intensity difference (middle panel minus left panel), with ddaC dendrites (arrow) and axon (arrowhead) marked. c, d, Similar experiments with 5 s green (546 nm; c) and ultraviolet light (365 nm; d) revealed ddaC activation by ultraviolet, but not green, light. Scale bar in a–d, 20 μm; colour scale in right panels of b–d shows dynamic range (0–4,095). e, Time course of somatic GCaMP3 signals of dorsal cluster neurons shown in a–d. Time frames are indicated. f, Summary of somatic fluorescence changes (Δ_F_/F) of dorsal cluster neurons in response to 5 s light stimulation, n =7–16. g, Example firing traces of ddaC in response to 5 s 470 nm blue light. h, Summary of firing frequency changes (average frequency of 5 s before light exposure subtracted from average frequency during 5 s of light exposure) of ddaC induced by white, 340, 380, 402, 470, 525 and 620 nm light. For clarity, significance is only shown for the 340 nm curve. Light intensity is reported as the log of (I normalized to _I_0 =1 mW mm−2). Green (525 nm) or red (620 nm) light has no effect (P >0.05). n =5–9. i, Effect of 1.4 mW mm−2 white light on ddaC, average frequencies of 5 s before (control) and during the 5 s of light exposure (light) are plotted. n =6. *P <0.05, **P <0.01, ***P <0.001; two-tailed paired _t_-test. All error bars indicate s.e.m.
Figure 3. Cell-autonomous activation of class IV dendritic arborization neurons by light
a, b, Quantification of somatic fluorescence changes (Δ_F_/F) in response to 5 s light and 100 μM allyl isothiocyanate (AITC) stimulation of cultured class IV (a) and III (b) dendritic arborization neurons; RFP signals serve as control. n =10–13 (light) and n =4 (AITC) in a, n =9 in b. c, Larva with class IV dendritic arborization neurons labelled with GFP by ppk-GAL4. Dendrites tile the body wall. Boxed area shows an abdominal hemi-segment; three dotted circles mark soma positions of D (dorsal, ddaC), L (lateral, V′ada) and V (ventral, VdaB) class IV dendritic arborization neurons, respectively. Up, dorsal; left, anterior. Scale bar, 200 μm. d, Illumination of dendrites within the dotted circle of GFP-labelled ddaC dendrites. Up, dorsal. Scale bar, 50 μm. e, Responses of ddaC with dendritic illumination. n =5. *P <0.05, **P <0.01, ***P <0.001; two-tailed paired _t_-test. All error bars indicate s.e.m.
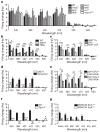
Figure 4. Gr28b and TrpA1 are essential for class IV dendritic arborization neuron light responses
a, No significant defects were detected between wild-type and mutants of known phototransduction molecules with 340, 380, 402, 470, or 620 nm light. n =5–10. b, Reduced light response of class IV dendritic arborization neurons in MiET1 and PBac larvae. n =8–29. c, Reduced light response of class IV dendritic arborization neurons in MiET1/deficiency larvae. n =5–12. d, Precise excision of MiET1 P-element insertion restores light response in class IV dendritic arborization neurons. n =6–9. e, Reduced light responses of class IV dendritic arborization neurons with Gr28b RNAi knockdown. n =5–8. f, Abolished light responses of class IV dendritic arborization neurons in _TrpA1_−/− mutants. n =8–13. g, MARCM analysis of TrpA1+/− and _TrpA1_−/− class IV dendritic arborization neurons’ response to light. n =5–8. For a–g, Light intensities (mW mm−2) are: 1.15 (340 nm), 5.79 (380 nm), 11.4 (402 nm), 52.8 (470 nm), 43.4 (525 nm), 29.6 (620 nm) and 94.7 (white). For a, b, c, e, *P <0.05, **P <0.01, ***P <0.001; one-way ANOVA followed by a Bonferroni post test; for d, f, g, *P <0.05, **P <0.01, ***P <0.001; two-tailed unpaired _t_-test. All error bars indicate s.e.m.
Figure 5. Class IV dendritic arborization neurons are the extra-ocular photoreceptors that contribute to light avoidance
a, b, Examples of larvae with either class IV dendritic arborization neurons ablated (a) or both Bolwig organs and class IV dendritic arborization neurons ablated (b) that failed to respond to white light (0.57 mW mm−2) applied from 0 to 5 s (dotted circle). Arrow indicates locomotion direction. Scale bar, 1 mm (a, b), shown at −2 s. c–g, Percentage of animals avoiding white light of different intensities (in mW mm−2: c, 0.088; d, 0.24; e, 0.57; f, 1.0; g, 1.67). Wild-type larvae, Bolwig-organ-ablated larvae (GMR-Hid), larvae with class IV dendritic arborization neurons ablated (ppk-GAL4; UAS-Hid, rpr) and larvae with both ablated (UAS-Hid, rpr; GMR-Hid; ppk-GAL4) were examined. h, Percentage of Bolwig-organ-ablated animals avoiding 0.25 mW mm−2 525 nm green light when class IV dendritic arborization neurons express ChR2 with or without dietary retinal. i, Percentage of animals avoiding white light at 1 mW mm−2. For c–i, controls are black bars. Twenty to forty animals were tested for each condition; *P <0.05, **P <0.01, ***P <0.001; two-tailed Fisher exact test. ChR2, channelrhodopsin-2; rpr, reaper; NS, not significant.
Comment in
- Neuroscience: Feel the light.
Garrity PA. Garrity PA. Nature. 2010 Dec 16;468(7326):900-1. doi: 10.1038/468900a. Nature. 2010. PMID: 21164472 No abstract available.
Similar articles
- Neuroscience: Feel the light.
Garrity PA. Garrity PA. Nature. 2010 Dec 16;468(7326):900-1. doi: 10.1038/468900a. Nature. 2010. PMID: 21164472 No abstract available. - Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster.
Rosenzweig M, Kang K, Garrity PA. Rosenzweig M, et al. Proc Natl Acad Sci U S A. 2008 Sep 23;105(38):14668-73. doi: 10.1073/pnas.0805041105. Epub 2008 Sep 11. Proc Natl Acad Sci U S A. 2008. PMID: 18787131 Free PMC article. - Light Spot-Based Assay for Analysis of Drosophila Larval Phototaxis.
Sun Y, Zhou P, Zhao Q, Gong Z. Sun Y, et al. J Vis Exp. 2019 Sep 27;(151). doi: 10.3791/60235. J Vis Exp. 2019. PMID: 31609336 - Temperature sensation in Drosophila.
Barbagallo B, Garrity PA. Barbagallo B, et al. Curr Opin Neurobiol. 2015 Oct;34:8-13. doi: 10.1016/j.conb.2015.01.002. Epub 2015 Jan 21. Curr Opin Neurobiol. 2015. PMID: 25616212 Free PMC article. Review. - The regulations of Drosophila phototransduction.
Leung HT, Shino S, Kim E. Leung HT, et al. J Neurogenet. 2012 Jun;26(2):144-50. doi: 10.3109/01677063.2011.650253. Epub 2012 Mar 15. J Neurogenet. 2012. PMID: 22420370 Review.
Cited by
- Multiple mechanisms of action of an extremely painful venom.
Borjon LJ, de Assis Ferreira LC, Trinidad JC, Šašić S, Hohmann AG, Tracey WD. Borjon LJ, et al. bioRxiv [Preprint]. 2024 Sep 12:2024.09.12.612741. doi: 10.1101/2024.09.12.612741. bioRxiv. 2024. PMID: 39314321 Free PMC article. Preprint. - Transsynaptic BMP Signaling Regulates Fine-Scale Topography between Adjacent Sensory Neurons.
Kaneko T, Li R, He Q, Yang L, Ye B. Kaneko T, et al. eNeuro. 2024 Aug 28;11(8):ENEURO.0322-24.2024. doi: 10.1523/ENEURO.0322-24.2024. Print 2024 Aug. eNeuro. 2024. PMID: 39137988 Free PMC article. - Altered circadian rhythm, sleep, and _rhodopsin 7_-dependent shade preference during diapause in Drosophila melanogaster.
Meyerhof GT, Easwaran S, Bontempo AE, Montell C, Montell DJ. Meyerhof GT, et al. Proc Natl Acad Sci U S A. 2024 Jul 2;121(27):e2400964121. doi: 10.1073/pnas.2400964121. Epub 2024 Jun 25. Proc Natl Acad Sci U S A. 2024. PMID: 38917005 Free PMC article. - Calcium plays an essential role in early-stage dendrite injury detection and regeneration.
Duarte VN, Lam VT, Rimicci DS, Thompson-Peer KL. Duarte VN, et al. Prog Neurobiol. 2024 Aug;239:102635. doi: 10.1016/j.pneurobio.2024.102635. Epub 2024 May 31. Prog Neurobiol. 2024. PMID: 38825174 Free PMC article. - Uncommon opsin's retinal isomer is involved in mammalian sperm thermotaxis.
Brandis A, Roy D, Das I, Sheves M, Eisenbach M. Brandis A, et al. Sci Rep. 2024 May 10;14(1):10699. doi: 10.1038/s41598-024-61488-3. Sci Rep. 2024. PMID: 38729974 Free PMC article.
References
- Steven DM. The dermal light sense. Biol Rev Camb Philos Soc. 1963;38:204–240. - PubMed
- Millott N. The dermal light sense. Symp Zool Soc Lond. 1968;23:1–36.
- Yoshida M. Handbook of Sensory Physiology. 7/6A. Springer; 1979. Extraocular photoreception; pp. 581–640.
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH084234/MH/NIMH NIH HHS/United States
- R37 NS040929/NS/NINDS NIH HHS/United States
- R37 NS040929-11/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases