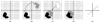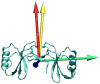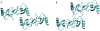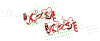Structural NMR of protein oligomers using hybrid methods - PubMed (original) (raw)
Review
Structural NMR of protein oligomers using hybrid methods
Xu Wang et al. J Struct Biol. 2011 Mar.
Abstract
Solving structures of native oligomeric protein complexes using traditional high-resolution NMR techniques remains challenging. However, increased utilization of computational platforms, and integration of information from less traditional NMR techniques with data from other complementary biophysical methods, promises to extend the boundary of NMR-applicable targets. This article reviews several of the techniques capable of providing less traditional and complementary structural information. In particular, the use of orientational constraints coming from residual dipolar couplings and residual chemical shift anisotropy offsets are shown to simplify the construction of models for oligomeric complexes, especially in cases of weak homo-dimers. Combining this orientational information with interaction site information supplied by computation, chemical shift perturbation, paramagnetic surface perturbation, cross-saturation and mass spectrometry allows high resolution models of the complexes to be constructed with relative ease. Non-NMR techniques, such as mass spectrometry, EPR and small angle X-ray scattering, are also expected to play increasingly important roles by offering alternative methods of probing the overall shape of the complex. Computational platforms capable of integrating information from multiple sources in the modeling process are also discussed in the article. And finally a new, detailed example on the determination of a chemokine tetramer structure will be used to illustrate how a non-traditional approach to oligomeric structure determination works in practice.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1
Steps of the grid search used to produce dimer models of Sr360 & SeR13. (a) Place monomer in the alignment tensor frame. (b) Create 2nd monomer by rotating the structure 180 degrees around one of the principal axes. (c) Translate the 2nd monomer in the plane perpendicular to the rotation axis. Discard models that have monomers too far apart or too close. (d) Perform energy minimization and MD to relax the interface and create better complementarity. (e) Evaluate the model based on experimental/simulated RDC correlation, RP score and VDW energy. Adapted from (Wang et al., 2008).
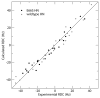
Figure 2
Correlation between experimental RDCs for both the wild type and E66S CCL5 and those calculated from the CCL5 dimer structure (PDB accession code 1U4L).
Figure 3
Orientation of the alignment tensor principal axes and the symmetry axis of the dimer. The symmetry axis is shown in gold; the Sxx axis of the tensor is shown in red; the Syy axis is shown in blue and the Szz axis is shown in green. The angle between the symmetry axis and the Sxx axis is 11 degrees.
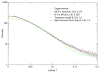
Figure 4
Experimental scattering curve for wild type CCL5 and the calculated scattering curves for the MCP-1 tetramer, the IP-10 tetramer, and a tretramer based on RDC and SAXS data. q is the magnitude of the momentum transfer vector, 4πsin(θ)/λ, where 2θ is the scattering angle and λ is the X-ray wavelength.
Figure 5
Steps in the grid search used in constructing the tetramer models of CCL5. (a) Place one dimeric unit in the alignment tensor frame. (b) Create 2nd dimer by rotating the structure 180 degrees around the dimeric symmetry axis. (c) Translate the 2nd dimer in the plane perpendicular to the rotation axis. Discard models that have the dimers too far apart or too close. (d) Perform energy minimization and MD to relax the interface and create better complementarity. (e) Evaluate the model based agreement between theoretical and experimental scattering curve and residue pairing score.
Figure 6
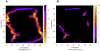
A) Contour plot of the scattering curve fitting χ values for models generated by the grid search. The four locations on the grid that produced better fitting models are 3×12, 54×45, 4×43, 61×18. B) Contour plot of the residue-pairing score of the models generated by the grid search.
Figure 7
Two models of the tetramer whose scattering profile showed good agreement with the experimental scattering curve of wild type CCL5. Model A is from grid 3×12 and model B is from grid 4×43.
Figure 8
Comparison of the grid search models with the ab initio shape generated from the scattering curve. The tetramer model A superimposed onto the dummy atom model calculated from the scattering curve (green spheres).
Figure 9
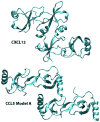
Comparison of the tetrameric interface of CXCL12 (PDB accession code 3HP3, upper panel) and Model A of the CCL5 tetramer model found by grid search.
Similar articles
- Oligomeric structure of the chemokine CCL5/RANTES from NMR, MS, and SAXS data.
Wang X, Watson C, Sharp JS, Handel TM, Prestegard JH. Wang X, et al. Structure. 2011 Aug 10;19(8):1138-48. doi: 10.1016/j.str.2011.06.001. Structure. 2011. PMID: 21827949 Free PMC article. - NMR approaches for structural analysis of multidomain proteins and complexes in solution.
Göbl C, Madl T, Simon B, Sattler M. Göbl C, et al. Prog Nucl Magn Reson Spectrosc. 2014 Jul;80:26-63. doi: 10.1016/j.pnmrs.2014.05.003. Epub 2014 May 23. Prog Nucl Magn Reson Spectrosc. 2014. PMID: 24924266 Review. - High-field EPR on membrane proteins - crossing the gap to NMR.
Möbius K, Lubitz W, Savitsky A. Möbius K, et al. Prog Nucl Magn Reson Spectrosc. 2013 Nov;75:1-49. doi: 10.1016/j.pnmrs.2013.07.002. Epub 2013 Jul 29. Prog Nucl Magn Reson Spectrosc. 2013. PMID: 24160760 Review. - Determination of the structures of symmetric protein oligomers from NMR chemical shifts and residual dipolar couplings.
Sgourakis NG, Lange OF, DiMaio F, André I, Fitzkee NC, Rossi P, Montelione GT, Bax A, Baker D. Sgourakis NG, et al. J Am Chem Soc. 2011 Apr 27;133(16):6288-98. doi: 10.1021/ja111318m. Epub 2011 Apr 5. J Am Chem Soc. 2011. PMID: 21466200 Free PMC article. - Transient protein-protein interactions visualized by solution NMR.
Liu Z, Gong Z, Dong X, Tang C. Liu Z, et al. Biochim Biophys Acta. 2016 Jan;1864(1):115-22. doi: 10.1016/j.bbapap.2015.04.009. Epub 2015 Apr 18. Biochim Biophys Acta. 2016. PMID: 25896389 Review.
Cited by
- Chemokine oligomerization in cell signaling and migration.
Wang X, Sharp JS, Handel TM, Prestegard JH. Wang X, et al. Prog Mol Biol Transl Sci. 2013;117:531-78. doi: 10.1016/B978-0-12-386931-9.00020-9. Prog Mol Biol Transl Sci. 2013. PMID: 23663982 Free PMC article. Review. - Protein-protein complexation in bioluminescence.
Titushin MS, Feng Y, Lee J, Vysotski ES, Liu ZJ. Titushin MS, et al. Protein Cell. 2011 Dec;2(12):957-72. doi: 10.1007/s13238-011-1118-y. Epub 2012 Jan 10. Protein Cell. 2011. PMID: 22231355 Free PMC article. Review. - Integrative structural modeling with small angle X-ray scattering profiles.
Schneidman-Duhovny D, Kim SJ, Sali A. Schneidman-Duhovny D, et al. BMC Struct Biol. 2012 Jul 16;12:17. doi: 10.1186/1472-6807-12-17. BMC Struct Biol. 2012. PMID: 22800408 Free PMC article. Review. - Oligomeric structure of the chemokine CCL5/RANTES from NMR, MS, and SAXS data.
Wang X, Watson C, Sharp JS, Handel TM, Prestegard JH. Wang X, et al. Structure. 2011 Aug 10;19(8):1138-48. doi: 10.1016/j.str.2011.06.001. Structure. 2011. PMID: 21827949 Free PMC article. - What's the defect? Using mass defects to study oligomerization of membrane proteins and peptides in nanodiscs with native mass spectrometry.
Townsend JA, Marty MT. Townsend JA, et al. Methods. 2023 Oct;218:1-13. doi: 10.1016/j.ymeth.2023.07.004. Epub 2023 Jul 22. Methods. 2023. PMID: 37482149 Free PMC article. Review.
References
- Al-Hashimi HM, Bolon PJ, Prestegard JH. Molecular symmetry as an aid to geometry determination in ligand protein complexes. J Magn Reson. 2000a;142:153–8. - PubMed
- Al-Hashimi HM, Valafar H, Terrell M, Zartler ER, Eidsness MK, Prestegard JH. Variation of molecular alignment as a means of resolving orientational ambiguities in protein structures from dipolar couplings. Journal of Magnetic Resonance. 2000b;143:402–406. - PubMed
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang WZ, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Rout MP, Sali A. Determining the architectures of macromolecular assemblies. Nature. 2007;450:683–694. - PubMed
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKRS: A RANTES, MIP-1 alpha, MIP-1 beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 GM074958/GM/NIGMS NIH HHS/United States
- P41 GM103390/GM/NIGMS NIH HHS/United States
- K99 GM088483-02/GM/NIGMS NIH HHS/United States
- U54-GM074958/GM/NIGMS NIH HHS/United States
- U54 GM094597/GM/NIGMS NIH HHS/United States
- K99 GM088483-01/GM/NIGMS NIH HHS/United States
- R00 GM088483/GM/NIGMS NIH HHS/United States
- U54 GM074958-05/GM/NIGMS NIH HHS/United States
- K99GM088483/GM/NIGMS NIH HHS/United States
- K99 GM088483/GM/NIGMS NIH HHS/United States
- P41-RR005351/RR/NCRR NIH HHS/United States
- P41 RR005351-21/RR/NCRR NIH HHS/United States
- P41 RR005351-20/RR/NCRR NIH HHS/United States
- P41 RR005351/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources