Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment - PubMed (original) (raw)
. 2011 Mar 22:1380:106-19.
doi: 10.1016/j.brainres.2010.11.032. Epub 2010 Nov 12.
Brian P Schoenfeld, Aaron J Bell, Paul Hinchey, Maria Kollaros, Michael J Gertner, Newton H Woo, Michael R Tranfaglia, Mark F Bear, R Suzanne Zukin, Thomas V McDonald, Thomas A Jongens, Sean M J McBride
Affiliations
- PMID: 21078304
- PMCID: PMC3050427
- DOI: 10.1016/j.brainres.2010.11.032
Pharmacological reversal of synaptic plasticity deficits in the mouse model of fragile X syndrome by group II mGluR antagonist or lithium treatment
Catherine H Choi et al. Brain Res. 2011.
Abstract
Fragile X syndrome is the leading single gene cause of intellectual disabilities. Treatment of a Drosophila model of Fragile X syndrome with metabotropic glutamate receptor (mGluR) antagonists or lithium rescues social and cognitive impairments. A hallmark feature of the Fragile X mouse model is enhanced mGluR-dependent long-term depression (LTD) at Schaffer collateral to CA1 pyramidal synapses of the hippocampus. Here we examine the effects of chronic treatment of Fragile X mice in vivo with lithium or a group II mGluR antagonist on mGluR-LTD at CA1 synapses. We find that long-term lithium treatment initiated during development (5-6 weeks of age) and continued throughout the lifetime of the Fragile X mice until 9-11 months of age restores normal mGluR-LTD. Additionally, chronic short-term treatment beginning in adult Fragile X mice (8 weeks of age) with either lithium or an mGluR antagonist is also able to restore normal mGluR-LTD. Translating the findings of successful pharmacologic intervention from the Drosophila model into the mouse model of Fragile X syndrome is an important advance, in that this identifies and validates these targets as potential therapeutic interventions for the treatment of individuals afflicted with Fragile X syndrome.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
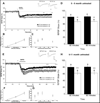
Figure 1. mGluR-LTD is enhanced in Fmr1 KO mice at 5–6 months of age
(A) In panels A–H Fmr1 KO (open circles or bars) and interleaved age-matched WT mice (filled squares or bars). Long-term depression of synaptic transmission was induced by brief bath application of the mGluR agonist DHPG (100 µM, 10 min). Mean fEPSP slopes (± SEM) are plotted as a percentage of average pre-induction baseline values. DHPG-induced mGluR-LTD was enhanced in 5–6 month old Fmr1 KO mice (n = 8 slices) compared to interleaved age-matched WT mice (n = 6 slices) at 60 minutes (WT 5–6 months: 82.9 ± 2.3%; Fmr1 KO 5–6 months: 68.3 ± 1.5%; p = 0.0009) and at 80 minutes (WT 5–6 months: 83.5 ± 2.8%; Fmr1 KO 5–6 months: 68.9 ± 1.6%; p = 0.0009) post-induction. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (B) Basal synaptic transmission is normal in Fmr1 KO mice at 5–6 months of age. Mean evoked fEPSP slopes (± SD) are plotted at three different stimulus intensities. Synaptic responses at threshold, half-maximal and maximal stimulus intensities were not significantly different between 5–6 month old Fmr1 KO (n = 8) and interleaved age-matched WT mice (n = 6). (C) Paired-pulse facilitation is normal in Fmr1 KO mice at 5–6 months of age. Synaptic responses to paired stimulation were evoked at interstimulus intervals ranging from 15 ms to 100 ms. Plotted are mean percent facilitation (± SD), as determined by calculating the ratio of the second fEPSP slope to the first fEPSP slope. At all interpulse intervals, no significant differences were observed in PPF between 5–6 month old Fmr1 KO mice (n = 8) and interleaved age-matched WT mice (n = 6). (D) Graphical representation of panel A at 60 and at 80 minutes after DHPG induction of LTD. ** represents p < 0.001. The number above each bar denotes the n. Color code as in panel A. (E) Long-term depression of synaptic transmission was induced and plotted as in panel A. DHPG-induced mGluR-LTD was enhanced in 9–11 month old Fmr1 KO mice (n = 8 slices) compared to interleaved age-matched WT mice (n = 9 slices) at 60 minutes (WT: 81.1 ± 3.5%; Fmr1 KO: 62.9 ± 4.1%; p = 0.0001) and at 80 minutes (WT: 81.9 ± 3.4%; Fmr1 KO: 63.5 ± 3.8%; p = 0.0001) post-induction. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (F) Basal synaptic transmission is normal in Fmr1 KO mice at 9–11 months of age and is plotted as in panel B. Synaptic responses at threshold, half-maximal and maximal stimulus intensities were not significantly different between 9–11 month old Fmr1 KO (n = 8 slices) and interleaved age-matched WT mice (n = 9 slices). (G) Paired-pulse facilitation is normal in Fmr1 KO mice at 9–11 months of age and is plotted as in panel C. No significant differences were observed in PPF between 9–11 month old Fmr1 KO mice (n = 8 slices) and interleaved age-matched WT mice (n = 9 slices). (H) Graphical representation of panel E at 60 and at 80 minutes after DHPG induction of LTD. *** represents p < 0.0001. Color code as in panel E.
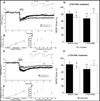
Figure 2. Long-term chronic treatment of WT mice with lithium does not alter mGluR-LTD, while in contrast it abrogates the enhanced mGluR-LTD in Fragile X mice
(A) mGluR-LTD is normal in aged WT after chronic long-term treatment with lithium. 5–6 week old WT mice were administered lithium-containing chow ad libitum throughout adulthood until 10–11 months of age. LTD was induced by brief bath application of the mGluR agonist DHPG (100 µM, 10 min). DHPG-induced mGluR-LTD was not significantly different between long-term lithium-treated WT mice (n = 8 slices, open circles, A–C) and interleaved age-matched vehicle-treated WT mice (n = 5 slices, filled squares, A–C) at 60 minutes (WT LT vehicle: 81.8 ± 2.9%; WT LT lithium: 82.2 ± 1.8%) or at 80 minutes (WT long-term vehicle: 81.1 ± 3.5%; WT long-term lithium: 84.1 ± 1.9%) post-induction. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (B) Basal synaptic transmission is not affected by chronic long-term lithium treatment in aged WT mice and is plotted as in Figure 1. (vehicle treated WT mice, n = 5; lithium treated WT mice, n = 8) (C) Paired-pulse facilitation is not affected by chronic long-term lithium treatment in aged WT mice and is plotted as in Figure 1. (vehicle treated WT mice, n = 5; lithium treated WT mice, n = 8) (D) The enhanced DHPG-induced LTD was abrogated in Fmr1 KO mice that were administered lithium-containing chow ad libitum beginning at 5–6 weeks of age throughout adulthood until 10–11 months of age. LTD was induced by brief application of the mGluR agonist DHPG (100 µM, 10 min). mGluR-LTD was significantly enhanced in long-term vehicle-treated Fmr1 KO mice (n = 5 slices, filled squares, D–F) compared to interleaved age-matched vehicle-treated WT mice (2A; n = 5 slices, filled squares) at 60 minutes (WT LT vehicle: 81.8 ± 2.9%; Fmr1 KO LT vehicle: 62.1 ± 4.5%; p = 0.0001) and at 80 minutes (WT LT vehicle: 81.1 ± 3.5%; Fmr1 KO LT vehicle: 61.8 ± 3.8%; p = 0.0002) post induction. Chronic long-term treatment of Fmr1 KO mice with lithium (n = 9 slices, open circles, D–F) abrogated the enhanced mGluR-LTD phenotype compared to vehicle-treated Fmr1 KO mice at 60 minutes (Fmr1 KO LT vehicle 62.1 ± 4.5%; Fmr1 KO LT lithium: 79.3 ± 1.4%; p = 0.0001) and at 80 minutes (Fmr1 KO LT vehicle: 61.8 ± 3.8%; Fmr1 KO LT lithium: 83.9 ± 1.4%; p = 0.0001) post-induction. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (E) Basal synaptic transmission is normal in long-term lithium treated Fmr1 KO mice at 10–11 months of age and is plotted as in Figure 1. (vehicle treated Fmr1 KO mice, n = 5; lithium treated Fmr1 KO mice, n = 9) (F) Paired-pulse facilitation is normal in long-term lithium treated Fmr1 KO mice at 10–11 months of age and is plotted as in Figure 1. (vehicle treated Fmr1 KO mice, n = 5; lithium treated Fmr1 KO mice, n = 9) (G) Chronic long-term treatment of Fmr1 KO mice with lithium beginning at 5–6 weeks old and continuing throughout adulthood until 10–11 months of age abrogates the enhanced mGluR-LTD phenotype. DHPG-LTD in WT (filled bars) and Fmr1 KO (open bars) mice treated with control chow or lithium chow at 60 minutes after induction as shown in Figures 2A and 2D. The asterisks *** represent p < 0.0001 for comparisons of drug versus vehicle treatment within the same genotype. The § represents a significant difference between WT and Fmr1 KO mice on vehicle control chow (p = 0.0001). The number above each bar denotes the n. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values. (H) DHPG-LTD in WT and Fmr1 KO mice treated with vehicle or lithium chow at 80 minutes after induction as shown in Figures 2A and 2D. The asterisks *** represent p < 0.0001 for comparisons of drug versus vehicle treatment within the same genotype. The § represents a significant difference between WT and Fmr1 KO mice on vehicle control chow (p = 0.0002). The number above each bar denotes the n. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values.
Figure 3. Chronic short-term treatment of WT mice with lithium does not alter mGluR-LTD, while in contrast it abrogates the enhanced mGluR-LTD in Fragile X mice
(A) mGluR-LTD is not changed in WT mice after chronic treatment with lithium. Two-month old WT mice were administered lithium-containing chow ad libitum until 4–5 months of age. LTD was induced by brief bath application of the mGluR agonist DHPG (100 µM, 10 min). DHPG-induced mGluR-LTD was not significantly different in the lithium-treated WT mice (n = 8 slices, open circles) compared to interleaved age-matched vehicle-treated WT mice (n = 6 slices, filled squares) at 60 minutes (WT vehicle: 78.7 ± 2.0%; WT lithium: 83.5 ± 2.1%) or at 80 minutes (WT vehicle: 78.3 ± 1.9%; WT lithium: 82.6 ± 1.6%) post-induction. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (B) Basal synaptic transmission is not affected by chronic short-term lithium treatment in WT mice. Mean evoked fEPSP slopes (± SD) are plotted at three different stimulus intensities. Synaptic responses at threshold, half-maximal and maximal stimulus intensities were not significantly different between lithium-treated WT mice (open circles) and interleaved age-matched vehicle-treated WT mice (filled squares). (vehicle treated WT mice, n = 6; lithium treated WT mice, n = 8) (C) Paired-pulse facilitation is not affected by chronic short-term lithium treatment in WT mice. Synaptic responses to paired stimulation were evoked at interstimulus intervals ranging from 15 ms to 100 ms. Plotted are mean percent facilitation (± SD), as determined by calculating the ratio of the second fEPSP slope to the first fEPSP slope. At all interpulse intervals, no significant differences were observed in PPF between lithium-treated WT mice (open circles) and interleaved age-matched vehicle-treated WT mice (filled squares). (vehicle treated WT mice, n = 6; lithium treated WT mice, n = 8) (D) Chronic short-term treatment of Fmr1 KO mice with lithium abrogates the enhanced mGluR-LTD phenotype. Eight week old Fmr1 KO mice were administered lithium-containing chow ad libitum until 4–5 months of age. LTD was induced by brief application of the mGluR agonist DHPG (100 µM, 10 min). mGluR-LTD was significantly enhanced in vehicle-treated Fmr1 KO mice (n = 7 slices, filled squares) compared to interleaved age-matched vehicle-treated WT mice (Figure 3A; n = 6 slices, filled squares) at 60 minutes (WT vehicle: 78.7 ± 2.0%; Fmr1 KO vehicle: 66.6 ± 3.1%; p = 0.0071) and at 80 minutes (WT vehicle: 78.3 ± 1.9%; Fmr1 KO vehicle: 66.3 ± 3.1%; p = 0.0077) post-induction. Chronic treatment of Fmr1 KO mice with lithium (n = 8 slices, open circles) abrogated the enhanced mGluR-LTD phenotype compared to vehicle-treated Fmr1 KO mice at 60 minutes (Fmr1 KO vehicle: 66.6 ± 3.1%; Fmr1 KO lithium: 81.8 ± 3.5%; p = 0.0003) and at 80 minutes (Fmr1 KO vehicle: 66.3 ± 3.1%; Fmr1 KO lithium: 80.8 ± 2.4%; p = 0.0006) post-induction. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (E) Basal synaptic transmission is not affected by chronic short-term lithium treatment in Fmr1 KO mice. Mean evoked fEPSP slopes (± SD) are plotted at three different stimulus intensities. Synaptic responses at threshold, half-maximal and maximal stimulus intensities were not significantly different between lithium-treated Fmr1 KO mice (open circles) and interleaved age-matched vehicle-treated Fmr1 KO mice (filled squares). (vehicle treated Fmr1 KO mice, n = 7; lithium treated Fmr1 KO mice, n = 8) (F) Paired-pulse facilitation is not affected by chronic short-term lithium treatment in Fmr1 KO mice. Synaptic responses to paired stimulation were evoked at interstimulus intervals ranging from 15 ms to 100 ms. Plotted are mean percent facilitation (± SD), as determined by calculating the ratio of the second fEPSP slope to the first fEPSP slope. At all interpulse intervals, no significant differences were observed in PPF between lithium-treated Fmr1 KO mice (open circles) and interleaved age-matched vehicle-treated Fmr1 KO mice (filled squares). (vehicle treated Fmr1 KO mice, n = 7; lithium treated Fmr1 KO mice, n = 8) (G) Chronic short-term treatment of Fmr1 KO mice with lithium beginning in adulthood until 4–5 months of age abrogates the enhanced mGluR-LTD phenotype. DHPG-LTD in WT (filled bars) and Fmr1 KO (open bars) mice treated with vehicle or lithium at 60 minutes after induction as shown in Figures 3A and 3D. The asterisks ** represent p < 0.001 for comparisons of drug versus vehicle treatment within the same genotype. The § represents a significant difference between WT and Fmr1 KO mice on vehicle control chow (p = 0.0071). The number above each bar denotes the n. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values. (H) DHPG-LTD in WT and Fmr1 KO mice treated with vehicle or lithium at 80 minutes after induction as shown in Figures 3A and 3D. The asterisks *** represent p < 0.0001 for comparisons of drug versus vehicle treatment within the same genotype. The § represents a significant difference between WT and Fmr1 KO mice on vehicle control chow (p = 0.0077). The number above each bar denotes the n. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values.
Figure 4. Chronic treatment of WT mice with the mGluR antagonist LY341495 enhances mGluR-LTD, while in contrast it abrogates the enhanced mGluR-LTD in Fragile X mice
(A) Chronic treatment of WT mice with the group II mGluR antagonist LY341495 enhances mGluR-LTD. Eight week old WT mice were administered daily injections of LY341495 for 8 weeks followed by a hiatus of 3–5 weeks. LTD was induced by brief bath application of the mGluR agonist DHPG (100 µM, 10 min). mGluR-LTD was significantly enhanced in LY341495-treated WT mice (n = 5 slices, open circles) compared to interleaved age-matched vehicle-treated WT mice (n = 5 slices, filled squares) at 60 minutes (WT vehicle: 83.1 ± 1.8%; WT LY341495: 69.1 ± 2.2%; p = 0.0001) and at 80 minutes (WT vehicle: 83.5 ± 1.8%; WT LY341495: 69.8 ± 2.8%; p = 0.0001) post-induction. Plotted are average fEPSP slope values (± SEM) as a percentage of average pre-induction baseline values. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 1.5 mV and 5 ms. (B) Basal synaptic transmission is not affected by chronic LY341495 treatment in WT mice. Mean evoked fEPSP slopes (± SD) are plotted at three different stimulus intensities. Synaptic responses at threshold, half-maximal and maximal stimulus intensities were not significantly different between the LY341495-treated WT mice (open circles) and interleaved age-matched vehicle-treated WT mice (filled squares). (vehicle treated WT mice, n = 5; LY341495 treated WT mice, n = 5) (C) Paired-pulse facilitation is normal in WT mice after chronic LY341495 treatment. Synaptic responses to paired stimulation were evoked at interstimulus intervals ranging from 15 ms to 100 ms. Plotted are mean percent facilitation (± SD), as determined by calculating the ratio of the second fEPSP slope to the first fEPSP slope. At all interpulse intervals, no significant differences were observed in PPF between LY341495-treated WT mice (open circles) and interleaved age-matched vehicle-treated WT mice (filled squares). (vehicle treated WT mice, n = 5; LY341495 treated WT mice, n = 5) (D) Chronic treatment of Fmr1 KO mice with the group II mGluR antagonist LY341495 abrogates the enhanced mGluR-LTD phenotype. Eight week old Fmr1 KO mice were administered daily injections of LY341495 for 8 weeks followed by a hiatus of 3–5 weeks. LTD was induced by brief bath application of the mGluR agonist DHPG (100 µM, 10 min). mGluR-LTD was significantly enhanced in vehicle-treated Fmr1 KO mice (n = 6 slices, filled squares) compared to interleaved age-matched vehicle-treated WT mice (Figure 4A; n = 4 slices, filled squares) at 60 minutes (WT vehicle: 83.1 ± 1.8%; Fmr1 KO vehicle: 72.6 ± 1.5%; p = 0.0005) and at 80 minutes (WT vehicle: 83.5 ± 1.8%; Fmr1 KO vehicle: 72.7 ± 1.3%; p = 0.0004) post-induction. Chronic treatment of Fmr1 KO mice with LY341495 (n = 7 slices, open circles) abrogated the enhanced mGluR-LTD phenotype compared to vehicle-treated Fmr1 KO mice at 60 minutes (Fmr1 KO vehicle: 72.6 ± 1.5%; Fmr1 KO LY341495: 84.1 ± 1.9%; p = 0.0001) and at 80 minutes (Fmr1 KO vehicle: 72.7 ± 1.3%; Fmr1 KO LY341495: 81.2 ± 2.0%; p = 0.0021) post-induction. Plotted are average fEPSP slopes (±SEM) as a percentage of average pre-induction baseline values. Representative traces of field potentials are from times indicated by the numbers on the graph (1 and 2). Calibration bars depict 2 mV and 5 ms. (E) Basal synaptic transmission is not affected by chronic LY341495 treatment in Fmr1 KO mice. Mean evoked fEPSP slopes (± SD) are plotted at three different stimulus intensities. Synaptic responses at threshold, half-maximal and maximal stimulus intensities were not significantly different between LY341495-treated Fmr1 KO mice (open circles) and interleaved age-matched vehicle-treated Fmr1 KO mice (filled squares). (vehicle treated Fmr1 KO mice, n = 6; LY341495 treated Fmr1 KO mice, n = 7) (F) Paired-pulse facilitation is not affected by chronic LY341495 treatment in Fmr1 KO mice. Synaptic responses to paired stimulation were evoked at interstimulus intervals ranging from 15 ms to 100 ms. Plotted are mean percent facilitation (± SD), as determined by calculating the ratio of the second fEPSP slope to the first fEPSP slope. At all interpulse intervals, no significant differences were observed in PPF between LY341495-treated Fmr1 KO mice (open circles) and interleaved age-matched vehicle-treated Fmr1 KO mice (filled squares). (vehicle treated Fmr1 KO mice, n = 6; LY341495 treated Fmr1 KO mice, n = 7) (G) Chronic treatment of Fmr1 KO mice with lithium throughout adulthood until 5–6 months of age abrogates the enhanced mGluR-LTD phenotype. DHPG-LTD in WT (filled bars) and Fmr1 KO (open bars) mice treated with vehicle or lithium at 60 minutes after induction as shown in Figures 4A and 4D. The asterisks *** represent p < 0.0001 for comparisons of drug versus vehicle treatment within the same genotype. The § represents a significant difference between WT and Fmr1 KO mice on vehicle control chow (p = 0.0005). The number above each bar denotes the n. Plotted are average fEPSP slopes (± SEM) as a percentage of average preinduction baseline values. (H) DHPG-LTD in WT and Fmr1 KO mice treated with vehicle or lithium at 80 minutes after induction as shown in Figures 4A and 4D. The asterisks ** represent p < 0.001 and *** represent p < 0.0001 for comparisons of drug versus vehicle treatment within the same genotype. The § represents a significant difference between WT and Fmr1 KO mice on vehicle control chow (p = 0.0004). The number above each bar denotes the n. Plotted are average fEPSP slopes (± SEM) as a percentage of average pre-induction baseline values.
Similar articles
- PDE-4 inhibition rescues aberrant synaptic plasticity in Drosophila and mouse models of fragile X syndrome.
Choi CH, Schoenfeld BP, Weisz ED, Bell AJ, Chambers DB, Hinchey J, Choi RJ, Hinchey P, Kollaros M, Gertner MJ, Ferrick NJ, Terlizzi AM, Yohn N, Koenigsberg E, Liebelt DA, Zukin RS, Woo NH, Tranfaglia MR, Louneva N, Arnold SE, Siegel SJ, Bolduc FV, McDonald TV, Jongens TA, McBride SM. Choi CH, et al. J Neurosci. 2015 Jan 7;35(1):396-408. doi: 10.1523/JNEUROSCI.1356-12.2015. J Neurosci. 2015. PMID: 25568131 Free PMC article. - Functional rescue of excitatory synaptic transmission in the developing hippocampus in Fmr1-KO mouse.
Meredith RM, de Jong R, Mansvelder HD. Meredith RM, et al. Neurobiol Dis. 2011 Jan;41(1):104-10. doi: 10.1016/j.nbd.2010.08.026. Epub 2010 Sep 15. Neurobiol Dis. 2011. PMID: 20817093 - Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome.
Nosyreva ED, Huber KM. Nosyreva ED, et al. J Neurophysiol. 2006 May;95(5):3291-5. doi: 10.1152/jn.01316.2005. Epub 2006 Feb 1. J Neurophysiol. 2006. PMID: 16452252 - Fragile X syndrome and targeted treatment trials.
Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Hagerman R, et al. Results Probl Cell Differ. 2012;54:297-335. doi: 10.1007/978-3-642-21649-7_17. Results Probl Cell Differ. 2012. PMID: 22009360 Free PMC article. Review. - Protein translation in synaptic plasticity: mGluR-LTD, Fragile X.
Waung MW, Huber KM. Waung MW, et al. Curr Opin Neurobiol. 2009 Jun;19(3):319-26. doi: 10.1016/j.conb.2009.03.011. Epub 2009 May 4. Curr Opin Neurobiol. 2009. PMID: 19411173 Free PMC article. Review.
Cited by
- Exploration of group II metabotropic glutamate receptor modulation in mouse models of Rett syndrome and MECP2 Duplication syndrome.
Vermudez SAD, Buch A, Weiss K, Gogliotti RG, Niswender CM. Vermudez SAD, et al. Neuropharmacology. 2022 May 15;209:109022. doi: 10.1016/j.neuropharm.2022.109022. Epub 2022 Mar 3. Neuropharmacology. 2022. PMID: 35248529 Free PMC article. - GSK-3_β_ at the Intersection of Neuronal Plasticity and Neurodegeneration.
Jaworski T, Banach-Kasper E, Gralec K. Jaworski T, et al. Neural Plast. 2019 May 2;2019:4209475. doi: 10.1155/2019/4209475. eCollection 2019. Neural Plast. 2019. PMID: 31191636 Free PMC article. Review. - The Role of mGluR Copy Number Variation in Genetic and Environmental Forms of Syndromic Autism Spectrum Disorder.
Wenger TL, Kao C, McDonald-McGinn DM, Zackai EH, Bailey A, Schultz RT, Morrow BE, Emanuel BS, Hakonarson H. Wenger TL, et al. Sci Rep. 2016 Jan 19;6:19372. doi: 10.1038/srep19372. Sci Rep. 2016. PMID: 26781481 Free PMC article. - Fragile X targeted pharmacotherapy: lessons learned and future directions.
Erickson CA, Davenport MH, Schaefer TL, Wink LK, Pedapati EV, Sweeney JA, Fitzpatrick SE, Brown WT, Budimirovic D, Hagerman RJ, Hessl D, Kaufmann WE, Berry-Kravis E. Erickson CA, et al. J Neurodev Disord. 2017 Jun 12;9:7. doi: 10.1186/s11689-017-9186-9. eCollection 2017. J Neurodev Disord. 2017. PMID: 28616096 Free PMC article. Review. - Testing Fmr1 KO Phenotypes in Response to GSK3 Inhibitors: SB216763 versus AFC03127.
Westmark PR, Garrone B, Ombrato R, Milanese C, Di Giorgio FP, Westmark CJ. Westmark PR, et al. Front Mol Neurosci. 2021 Oct 7;14:751307. doi: 10.3389/fnmol.2021.751307. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34690696 Free PMC article.
References
- Acharya JK, et al. Synaptic defects and compensatory regulation of inositol metabolism in inositol polyphosphate 1-phosphatase mutants. Neuron. 1998;20:1219–1229. - PubMed
- Altinbilek B, Manahan-Vaughan D. Antagonism of group III metabotropic glutamate receptors results in impairment of LTD but not LTP in the hippocampal CA1 region, and prevents long-term spatial memory. Eur J Neurosci. 2007;26:1166–1172. - PubMed
- Altinbilek B, Manahan-Vaughan D. A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience. 2009;158:149–158. - PubMed
- Bakker CE, Oostra BA. Understanding fragile X syndrome: insights from animal models. Cytogenet Genome Res. 2003;100:111–123. - PubMed
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS046573-03/NS/NINDS NIH HHS/United States
- R01 NS046573/NS/NINDS NIH HHS/United States
- R01 NS046573-04/NS/NINDS NIH HHS/United States
- R01 GM086902-05A2/GM/NIGMS NIH HHS/United States
- AS2087/Autism Speaks/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- R01 GM086902/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous