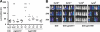Impact of glutamine transporters on pneumococcal fitness under infection-related conditions - PubMed (original) (raw)
Impact of glutamine transporters on pneumococcal fitness under infection-related conditions
Tobias Härtel et al. Infect Immun. 2011 Jan.
Abstract
The genomic analysis of Streptococcus pneumoniae predicted six putative glutamine uptake systems, which are expressed under in vitro conditions, as shown here by reverse transcription-PCR. Four of these operons consist of glnHPQ, while two lack glnH, which encodes a soluble glutamine-binding protein. Here, we studied the impact of two of these glutamine ATP-binding cassette transporters on S. pneumoniae D39 virulence and phagocytosis, which consist of GlnQ and a translationally fused protein of GlnH and GlnP. Mice infected intranasally with D39Δgln0411/0412 showed significantly increased survival times and a significant delay in the development of pneumococcal pneumonia compared to those infected with D39, as observed in real time using bioluminescent pneumococci. In a mouse sepsis model, the mutant D39Δgln0411/0412 showed only moderate but significant attenuation. In contrast, the D39Δgln1098/1099 knockout strain was massively attenuated in the pneumonia and septicemia mouse infection model. To cause pneumonia or sepsis with D39Δgln1098/1099, infection doses 100- to 10,000-fold higher than those used for wild-type strain D39 were required. In an experimental mouse meningitis model, D39Δgln1098/1099 produced decreased levels of white blood cells in cerebrospinal fluid and showed decreased numbers of bacteria in the bloodstream compared to D39 and D39Δgln0411/0412. Phagocytosis experiments revealed significantly decreased intracellular survival rates of mutants D39Δgln1098/1099 and D39Δgln0411/0412 compared to wild-type D39, suggesting that the deficiency of Gln uptake systems impairs resistance to oxidative stress. Taken together, our results demonstrate that both glutamine uptake systems are required for full virulence of pneumococci but exhibit different impacts on the pathogenesis of pneumococci under in vivo conditions.
Figures
FIG. 1.
Analysis of gln gene expression in S. pneumoniae D39 and isogenic gln mutants. (A) Genetic organization of operons encoding the putative glutamine transporters in S. pneumoniae D39 and construction of gln mutants in spd0411/0412 and spd1098/1099. (B) RT-PCR of glnQ genes of S. pneumoniae wild-type D39 and gln mutants D39Δ_gln0411_ and D39Δ_gln1098_/1099. cDNAs of the different strains were used as the templates in PCRs with gene-specific primers (Table 2). (C) Northern blot analysis of glnQ0411 and glnQ1098 mRNA levels in total RNA preparations of S. pneumoniae wild-type (WT) D39 and Δ_gln0411_ and Δ_gln1098_/1099 mutants. The blots were incubated with a DIG-labeled DNA probe (gln1098/1099) and a DIG-labeled RNA probe (gln0411/0412), respectively. nt, nucleotide. (D) Comparative transcript length analysis of the putative gln1099/1100 operon of wild-type D39 and mutant D39Δ_gln1098_ by RT-PCR. (Top) PCR fragments generated from cDNAs of both strains are shown for the 5′ end of the putative operon (lanes 1), the gln Q gene (lanes 2), the region containing the glnQ gene and the 5′ end of the zwf gene (lanes 3), the 5′ end of the zwf gene (lanes 4), and the enolase housekeeping gene (lanes 5). (Bottom) Localization of primers in the gln1099/1100 operon of S. pneumoniae D39. In the D39Δ_gln1098_ mutant, the sequence between primers 173 and 459 was replaced with the ermB gene cassette (panel A). The quality of the cDNA preparations was verified by expression analysis of the eno gene (primers SB80/SB81). The purity of the RNA was assessed by using RNA as the template in PCRs, which showed no PCR products (data not shown). (E) Gene expression levels of spd1098/1099 and spd1100 (zwf) in S. pneumoniae D39Δ_gln1098_/1099 as analyzed by quantitative RT-PCR (top) and RT-PCR (bottom). The results for each transcript in the quantitative RT-PCR are presented as the relative quantity (RQ) of expression in S. pneumoniae wild-type D39. The relative change in the expression of each gene was calculated from 2−ΔΔ_CT_ by using the genes for enolase (spd1012) and GAPDH (spd1823) as internal controls. The experiment was repeated three times, and one representative result is shown. The RT-PCR for spd1100 (zwf) was generated by using serial dilutions (undiluted to 1:128) of S. pneumoniae wild-type D39 and D39Δ_gln1098_/1099 mutant cDNAs as templates, respectively.
FIG. 2.
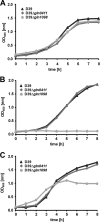
Growth behavior of pneumococcal gln mutants. S. pneumoniae strain D39 and its isogenic mutants gln0411 and gln1098 were cultured in THY (A), in CDM (B), or in CDM supplemented with 2% Casitone (C). Representative growth curves are shown.
FIG. 3.
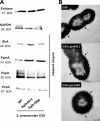
Influence of Gln deficiencies on the expression of pneumococcal surface-exposed virulence proteins and capsule. (A) The production of surface-exposed virulence proteins (SlrA, PpmA, PspA, and PspC) and glycolytic enzymes (GAPDH and enolase) in wild-type (WT) strain D39 and its isogenic mutants D39Δ_gln0411_ and D39Δ_gln1098_ was assessed by immunoblotting using protein-specific antibodies. Eno, enolase; PpmA, putative proteinase maturation protein A; SlrA, streptococcal lipoprotein rotamase A; PspA, pneumococcal surface protein A; PspC, pneumococcal surface protein C. (B) Capsular polysaccharide of D39 and its isogenic gln mutants shown by TEM after LRR fixation as described in Materials and Methods. Black arrows show the presence of polysaccharide capsule in all three samples. Bars, 200 nm.
FIG. 4.
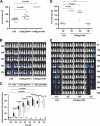
Impact of glutamine uptake systems on pneumococcal virulence. (A) Survival of CD-1 mice after intranasal infection with pneumococci. CD-1 mice (n = 12) were infected intranasally with a dose of 1 × 107 CFU of either wild-type strain D39 or its isogenic gln0411 or gln1098 mutant. (B) Real-time bioluminescent optical imaging of pneumococcal dissemination after intranasal infection of mice. CD-1 mice (n = 6) were intranasally infected with 1 × 107 CFU of wild-type D39 or its isogenic gln0411 and gln1098 mutants, respectively. Dissemination of pneumococci in this acute-pneumonia model was monitored for up to 144 h. (C) Determination of bioluminescent intensity at the indicated time points. Quantification of the total photon emission per second was done every 6 h by measuring the bioluminescence of CD-1 mice (n = 6) infected with wild-type D39 or its isogenic gln0411 or gln1098 mutant using the IVIS Lumina System. Data were evaluated using GraphPad Prism 4.0 software and are represented in a box whisker graph. (D) CD-1 mice (n = 6) were infected intranasally with a dose of 1 × 107 CFU of wild-type strain D39 or its isogenic gln1098 mutant using infection doses of 1 × 107, 1 × 108, or 1 × 109 CFU. n.s., not significant. (E) Monitoring of pneumococcal dissemination in the host after infection in a dose-dependent manner. Bioluminescent image analysis of CD-1 mice (n = 6) infected intranasally with a dose of 1 × 107 CFU of wild-type strain D39 or its isogenic gln1098 mutant.
FIG. 5.
Influence of glutamine uptake systems in a septicemia mouse infection model. (A) CD-1 mice (n = 6 for each group) were infected i.p. with a dose of 5 × 103 CFU of wild-type strain D39 or with 5 × 103, 1 × 104 or 1 × 105 CFU of mutant D39Δ_gln0411_. In parallel, mice were infected i.p. with doses ranging from 1 × 104 to 1 × 108 CFU of mutant strain D39Δ_gln1098_. The vitality of mice was observed every 3 h, and mice that died were scarified. (B) Bioluminescent image analysis of CD-1 mice (n = 6) infected i.p. with wild-type strain D39 or the mutants D39Δ_gln0411_ and D39Δ_gln1098_, respectively. The data from the time point shown indicate the massive differences in pneumococcal dissemination between the groups.
FIG. 6.
Role of glutamine uptake systems in an experimental mouse meningitis model. (A) Cerebrospinal fluid pleocytosis. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411 (n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows mean WBC counts per μl CSF, as a marker for an inflammatory response, 24 h after injection. The experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. n.s., not significant. (B) Blood bacterial titers. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411 (n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows mean counts (log CFU/ml) of bacteria in murine blood, as a marker of bacterial migration from the subarachnoid space into blood, 24 h after injection. Experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. Experimental details are described in Materials and Methods. (C) Hearing threshold clicks. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411(n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows the mean lowest stimulus intensity that elicited ABRs 24 h after injection. Experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. Experimental details are described in Materials and Methods. (D) Clinical scores. Different numbers of C57BL/6 mice were injected intracisternally with 1 × 107 CFU of wild-type strain D39 (n = 5), its gln0411(n = 6) or gln1098 (n = 6) mutant, or the same volume of PBS (n = 4) as a control. The histogram shows the mean clinical scores 24 h after injection. Experimental standard deviations are shown as bars, and two-tailed Student _t_-test analyses were performed on data sets. Experimental details are described in Materials and Methods. (E) Presence and localization of inflammatory cells in cochleae. Shown are dissected and H&E-stained cochleae of infected mice. A representative selection of images is displayed. Wild-type strain D39 or its gln0411 or gln1098 mutant was used for infection, or the same volume of PBS was used as a control. Black arrows show the recruitment of granulocytes into the scala tympani (*). The presence of granulocytes can lead to severe granulocytic labyrinthitis.
FIG. 7.
Impact of Gln deficiencies on phagocytosis and intracellular killing. (A) Numbers of viable bacteria after J774A.1 phagocytosis assay. Murine macrophages (105) were infected with a multiplicity of infection of 50 pneumococci of strain D39 or its gln0411 or gln1098 mutant. Viable invasive bacterial numbers were determined by colony counting after 5, 15, or 30 min of infection. The histogram shows the mean values ± standard deviation of three independent experiments. n.s., not significant. (B) Immunofluorescence microscopy of host cell-attached and intracellular pneumococci. J774A.1 murine macrophages were infected with pneumococcal strain D39 or its gln0411 or gln1098 mutant for 5, 30, or 60 min. Intracellular pneumococci were stained with Alexa568 (red), whereas adherent bacteria appear yellow (Alexa488 and Alexa568). Bars represent 10 μm. (C) Live/dead staining of phagocytosed pneumococci. J774A.1 murine macrophages were infected for 5, 30, or 60 min with wild-type D39 or its isogenic mutant D39Δ_gln0411_ or D39Δ_gln1098_. Viable bacteria are only stained with SYTO9 (green fluorescence), while dead bacteria appear red fluorescent as stained with propidium iodide. Higher magnifications of selected areas 60 min postinfection are shown on the right. Viable bacteria are indicated by arrows, and dead bacteria are indicated by arrowheads.
Similar articles
- The pavA gene of Streptococcus pneumoniae encodes a fibronectin-binding protein that is essential for virulence.
Holmes AR, McNab R, Millsap KW, Rohde M, Hammerschmidt S, Mawdsley JL, Jenkinson HF. Holmes AR, et al. Mol Microbiol. 2001 Sep;41(6):1395-408. doi: 10.1046/j.1365-2958.2001.02610.x. Mol Microbiol. 2001. PMID: 11580843 - Site-specific contributions of glutamine-dependent regulator GlnR and GlnR-regulated genes to virulence of Streptococcus pneumoniae.
Hendriksen WT, Kloosterman TG, Bootsma HJ, Estevão S, de Groot R, Kuipers OP, Hermans PW. Hendriksen WT, et al. Infect Immun. 2008 Mar;76(3):1230-8. doi: 10.1128/IAI.01004-07. Epub 2008 Jan 3. Infect Immun. 2008. PMID: 18174343 Free PMC article. - Regulation of the arginine deiminase system by ArgR2 interferes with arginine metabolism and fitness of Streptococcus pneumoniae.
Schulz C, Gierok P, Petruschka L, Lalk M, Mäder U, Hammerschmidt S. Schulz C, et al. mBio. 2014 Dec 23;5(6):e01858-14. doi: 10.1128/mBio.01858-14. mBio. 2014. PMID: 25538192 Free PMC article. - Switch from planktonic to sessile life: a major event in pneumococcal pathogenesis.
Oggioni MR, Trappetti C, Kadioglu A, Cassone M, Iannelli F, Ricci S, Andrew PW, Pozzi G. Oggioni MR, et al. Mol Microbiol. 2006 Sep;61(5):1196-210. doi: 10.1111/j.1365-2958.2006.05310.x. Mol Microbiol. 2006. PMID: 16925554 Free PMC article. - Streptococcus pneumoniae two-component regulatory systems: The interplay of the pneumococcus with its environment.
Gómez-Mejia A, Gámez G, Hammerschmidt S. Gómez-Mejia A, et al. Int J Med Microbiol. 2018 Aug;308(6):722-737. doi: 10.1016/j.ijmm.2017.11.012. Epub 2017 Nov 26. Int J Med Microbiol. 2018. PMID: 29221986 Review.
Cited by
- Bacterial surface lipoproteins mediate epithelial microinvasion by Streptococcus pneumoniae.
Chan JM, Ramos-Sevillano E, Betts M, Wilson HU, Weight CM, Houhou-Ousalah A, Pollara G, Brown JS, Heyderman RS. Chan JM, et al. Infect Immun. 2024 May 7;92(5):e0044723. doi: 10.1128/iai.00447-23. Epub 2024 Apr 17. Infect Immun. 2024. PMID: 38629841 Free PMC article. - Amino Acid Starvation-Induced Glutamine Accumulation Enhances Pneumococcal Survival.
Zhang C, Liu Y, An H, Wang X, Xu L, Deng H, Wu S, Zhang JR, Liu X. Zhang C, et al. mSphere. 2023 Jun 22;8(3):e0062522. doi: 10.1128/msphere.00625-22. Epub 2023 Apr 5. mSphere. 2023. PMID: 37017541 Free PMC article. - Streptococcus suis TrpX is part of a tryptophan uptake system, and its expression is regulated by a T-box regulatory element.
Dresen M, Schaaf D, Arenas J, de Greeff A, Valentin-Weigand P, Nerlich A. Dresen M, et al. Sci Rep. 2022 Aug 17;12(1):13920. doi: 10.1038/s41598-022-18227-3. Sci Rep. 2022. PMID: 35978073 Free PMC article. - Comprehensive Analysis Reveals the Genetic and Pathogenic Diversity of Ralstonia solanacearum Species Complex and Benefits Its Taxonomic Classification.
Geng R, Cheng L, Cao C, Liu Z, Liu D, Xiao Z, Wu X, Huang Z, Feng Q, Luo C, Chen Z, Zhang Z, Jiang C, Ren M, Yang A. Geng R, et al. Front Microbiol. 2022 May 6;13:854792. doi: 10.3389/fmicb.2022.854792. eCollection 2022. Front Microbiol. 2022. PMID: 35602040 Free PMC article. - Investigating Differential Expressed Genes of Limosilactobacillus reuteri LR08 Regulated by Soybean Protein and Peptides.
Zhu S, Zhang Y, Wang J, Zhang C, Liu X. Zhu S, et al. Foods. 2022 Apr 26;11(9):1251. doi: 10.3390/foods11091251. Foods. 2022. PMID: 35563974 Free PMC article.
References
- Benton, K. A., J. C. Paton, and D. E. Briles. 1997. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb. Pathog. 23:201-209. - PubMed
- Bergmann, S., and S. Hammerschmidt. 2006. Versatility of pneumococcal surface proteins. Microbiology 152:295-303. - PubMed
- Bergmann, S., D. Wild, O. Diekmann, R. Frank, D. Bracht, G. S. Chhatwal, and S. Hammerschmidt. 2003. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. 49:411-423. - PubMed
- Brown, J. S., S. M. Gilliland, and D. W. Holden. 2001. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol. Microbiol. 40:572-585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases