miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor - PubMed (original) (raw)
miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor
Nora E Renthal et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Throughout most of pregnancy, uterine quiescence is maintained by increased progesterone receptor (PR) transcriptional activity, whereas spontaneous labor is initiated/facilitated by a concerted series of biochemical events that activate inflammatory pathways and have a negative impact on PR function. In this study, we uncovered a previously undescribed regulatory pathway whereby micro-RNAs (miRNAs) serve as hormonally modulated and conserved mediators of contraction-associated genes in the pregnant uterus in the mouse and human. Using miRNA and gene expression microarray analyses of uterine tissues, we identified a conserved family of miRNAs, the miR-200 family, that is highly induced at term in both mice and humans as well as two coordinately down-regulated targets, zinc finger E-box binding homeobox proteins ZEB1 and ZEB2, which act as transcriptional repressors. We also observed up-regulation of the miR-200 family and down-regulation of ZEB1 and ZEB2 in two different mouse models of preterm labor. We further demonstrated that ZEB1 is directly up-regulated by the action of progesterone (P(4))/PR at the ZEB1 promoter. Excitingly, we observed that ZEB1 and ZEB2 inhibit expression of the contraction-associated genes, oxytocin receptor and connexin-43, and block oxytocin-induced contractility in human myometrial cells. Together, these findings implicate the miR-200 family and their targets, ZEB1 and ZEB2, as unique P(4)/PR-mediated regulators of uterine quiescence and contractility during pregnancy and labor and shed light on the molecular mechanisms involved in preterm birth.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
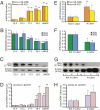
miR-200b/429, ZEB1, and ZEB2, and contraction-associated genes are coordinately regulated during late gestation. Mature miR-200b and miR-429 are significantly up-regulated (A) and ZEB1 and ZEB2 mRNA are significantly down-regulated (B) across late gestation in the mouse myometrium, beginning at 17.5 dpc. mmu, Mus musculus. (C) ZEB1 protein in nuclear extracts of murine myometrium mirrors the decline in ZEB1 mRNA. β-actin was used as a loading control. The immunoblot shown is representative of three independent gestational series of mice. Densitometry analysis of all three series revealed a significant decrease in ZEB1 protein between 15.5 dpc and labor (Student’s t test, P < 0.05; n = 3 mice per group). (D) Contraction-associated genes CXN-43 and OXTR were significantly up-regulated in the same samples as in A and B, beginning at 18.5 dpc. Expression of each miRNA/mRNA was determined by qRT-PCR, normalized to GAPDH/U6, and expressed as the fold increase over 15.5 dpc. Mean ± SEM values are shown. For one-way ANOVA, miR-200b: F(4,20) = 14.82, P < 0.0001; miR-429: F(4,20) = 17.49, P < 0.0001; ZEB1: F(4,20) = 12.71, P < 0.0001; ZEB2: F(4,20) = 5.37, P = 0.004; CXN-43: F(4,20) = 12.93, P < 0.0001; OXTR: F(4,20) = 11.03, P < 0.0001. (Multiple comparison test compared with 15.5 dpc: *P < 0.05, **P < 0.01; n = 10 mice each for 15.5 dpc and laboring groups, 5 mice each for 16.5–18.5 dpc.) miR-200b/429 were significantly up-regulated (E) and ZEB1 and ZEB2 mRNA were significantly down-regulated (F) in laboring myometrium as compared with nonlaboring controls. hsa, Homo sapiens. (G) ZEB1 protein expression was decreased in nuclear extracts of laboring myometrium. β-actin was used as a loading control. The immunoblot shown is representative of three replicate experiments. Densitometry analysis of all blots comparing laboring myometrium with nonlaboring controls revealed a significant decrease in ZEB1 protein in labor (Student’s t test, P < 0.05; n = 9 per group). (H) CXN-43 and OXTR in the same samples as in E and F were significantly up-regulated in laboring myometrium. Expression of each miRNA/mRNA was determined by qRT-PCR, normalized to U6/h36B4, and expressed as the fold increase over nonlaboring controls. Mean ± SEM values are shown (Student’s t test, *P < 0.05; n = 14 for laboring myometrium, n = 23 for nonlaboring controls).
Fig. 2.
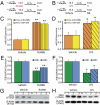
miR-200b/429 are up-regulated and ZEB1 and ZEB2 are down-regulated in two models of preterm labor. Diagram of RU486 treatment (A) or LPS treatment (B) to induce preterm labor. Animals were injected with a single s.c. injection of RU486 (200 μg) or intraamniotic injections of 1.5 μg of LPS into each sac at 15.5 dpc. IL, laboring myometrium; NIL, nonlaboring control. Mice were considered in labor on birth of one pup. miR-200b/429 are significantly up-regulated in RU486-induced (C) and LPS-induced (D) preterm labor. mmu, Mus musculus. ZEB1 and ZEB2 mRNA levels in the same tissue samples as in C and D are decreased with RU486-induced (E) or LPS-induced (F) preterm labor. Expression of each miRNA/mRNA was determined by qRT-PCR, normalized to U6/GAPDH, and expressed as the fold change over vehicle-treated controls. Mean ± SEM values are shown (Student’s t test, *P < 0.05, **P < 0.01; n = 7 per group). Experiments were repeated twice with similar results. ZEB1 protein expression in nuclear extracts of murine myometrium is decreased in association with RU486-induced (G) or LPS-induced (H) preterm labor. β-actin was used as a loading control. Densitometry analysis of blots comparing uterine nuclear extracts from RU486- and LPS-treated mice with vehicle-injected controls revealed a significant reduction in ZEB1 protein with preterm labor (Student’s t test, *P < 0.05; n = 7 mice per group).
Fig. 3.
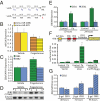
P4 affects the miR-200-ZEB contractile axis via direct induction of ZEB1 expression. (A) Diagram depicting P4 injection of mice in late gestation. IL, laboring myometrium; NIL, nonlaboring control. (B) miR-200b/429 are only modestly down-regulated by exogenous P4 treatment during late gestation. (C) ZEB1 but not ZEB2 mRNA is significantly increased by P4 treatment (same samples as in B). Expression of each miRNA/mRNA was determined by qRT-PCR, normalized to U6/GAPDH, and depicted as the fold increase over vehicle-treated controls. Mean ± SEM values are shown (Student’s t test, *P < 0.05; n = 7 mice per group). Data are representative of three similar experiments. (D) ZEB1 protein in myometrial nuclear extracts is increased by P4 treatment. β-actin was used as a loading control. Densitometry analysis of blots comparing P4-treated mice with vehicle controls revealed that P4 causes a significant increase in ZEB1 protein (Student’s t test, P < 0.05; n = 7 mice per group). (E) ZEB1 but not ZEB2 mRNA levels are induced in T74D cells treated with 10−7 M P4 for 12 or 24 h. Expression of each gene was determined by qRT-PCR, normalized to h36B4, and expressed as the fold increase over vehicle-treated control cells. Data are the mean ± SD values from three replicate experiments (Student’s t test, *P < 0.05, **P < 0.01). (F) P4 acting via PR induces ZEB1 promoter activity. HEK293 cells were transiently transfected with a ZEB1-Luciferase reporter construct containing 978 bp of the 5′-flanking sequence from the hZEB1 gene and with empty expression vector (control) or with a CMV expression vector containing the wild-type mouse PR-B isoform or PR-B containing a mutation in the DNA-binding domain (mutPR-BDBD). The cells were cultured with or without P4 (10−7 M) for 24 h, and luciferase activity was assayed, normalized to β-gal, and expressed as the fold increase over vector-transfected control cells. Data are the mean ± SD values from three replicate experiments (Student’s t test, *P < 0.05). (G) Overexpression of ZEB1 causes induction of ZEB2 mRNA expression. Primary murine myometrial cells infected with recombinant adenoviruses expressing CMV/ZEB1 manifested induction of endogenous ZEB2 mRNA after 72 and 96 h. Expression of ZEB2 was determined by qRT-PCR, normalized to GAPDH, and expressed as the fold increase over ZEB2 expression in cells transduced with β-gal–expressing adenoviruses. Data are the mean ± SD values from two replicate experiments (Student’s t test, *P < 0.05).
Fig. 4.
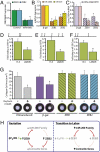
Overexpression of miR-200b/429 mimics, ZEB1, and ZEB2 regulates contraction-associated genes and human myometrial cell contractility. (A) Transfection of hTERT-HM cells with miRmimics of miR-200b/429 causes a significant reduction in endogenous ZEB1 and ZEB2 within 24 h. (B) Primary murine myometrial cells were infected with recombinant adenoviruses expressing ZEB1, ZEB2, or β-gal (control); levels of miR-200b and miR-429 were analyzed by qRT-PCR 96 h posttransduction. (C) hTERT-HM cells were infected with recombinant adenoviruses expressing ZEB1, ZEB2, or β-gal (control); CXN-43 and OXTR mRNA levels were analyzed by qRT-PCR 48 h posttransduction. The levels of each mRNA/miRNA were normalized to h36B4/U6 and expressed as the fold change over control. Data are the mean ± SD values of three replicate experiments (Student’s t test, *P < 0.05, **P < 0.01). ChIP studies of endogenous ZEB1 binding to the promoters of its targets reveals robust binding to CXN-43 (D), OXTR (E), and the miR-200b-a-429 cluster (F). Binding of endogenous ZEB1 was determined by qPCR, normalized to input, and expressed as the fold increase over binding by IgG. Data shown are the mean ± SEM values of two replicate experiments (Student’s t test, *P < 0.05; n = 10 mice per group). Diagrammed above each graph are the locations of E-boxes within the gene promoter. (G) Overexpression of ZEB1 and ZEB2 in hTERT-HM cells inhibits oxytocin-mediated contraction. Cells were infected with recombinant adenoviruses expressing ZEB1, ZEB2, or β-gal, (control) and cultured for 48 h. These, as well as uninfected cells, were embedded into collagen gel matrices and treated with or without oxytocin (10 nM). Contraction of the gels was assessed after 24 h by measurement and calculation of mean gel area (mm2). A representative gel is shown below the data for each group. Data shown are the mean ± SD values from three replicate experiments (Student’s t test, *P < 0.05). (H) During pregnancy, P4 and PR increase expression of ZEB1, which acts to suppress the miR-200 family as well as contraction-associated genes. Decreased expression of the miR-200 family relieves suppression of ZEB2 (as well as ZEB1), resulting in further down-regulation of contractile genes. Near term, a decrease in circulating P4 and/or a decrease in PR function results in down-regulation of ZEB1 expression, and, in turn, up-regulation of the miR-200 family, further suppressing ZEB1 and ZEB2. This removes the brakes from contractile gene expression, resulting in increased uterine contractility and labor.
Similar articles
- The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor.
Williams KC, Renthal NE, Gerard RD, Mendelson CR. Williams KC, et al. Mol Endocrinol. 2012 Nov;26(11):1857-67. doi: 10.1210/me.2012-1199. Epub 2012 Sep 12. Mol Endocrinol. 2012. PMID: 22973051 Free PMC article. - How does progesterone relax the uterus in pregnancy?
Zakar T, Mesiano S. Zakar T, et al. N Engl J Med. 2011 Mar 10;364(10):972-3. doi: 10.1056/NEJMcibr1100071. N Engl J Med. 2011. PMID: 21388317 No abstract available. - MicroRNA-200a serves a key role in the decline of progesterone receptor function leading to term and preterm labor.
Williams KC, Renthal NE, Condon JC, Gerard RD, Mendelson CR. Williams KC, et al. Proc Natl Acad Sci U S A. 2012 May 8;109(19):7529-34. doi: 10.1073/pnas.1200650109. Epub 2012 Apr 23. Proc Natl Acad Sci U S A. 2012. PMID: 22529366 Free PMC article. - MicroRNAs--mediators of myometrial contractility during pregnancy and labour.
Renthal NE, Williams KC, Mendelson CR. Renthal NE, et al. Nat Rev Endocrinol. 2013 Jul;9(7):391-401. doi: 10.1038/nrendo.2013.96. Epub 2013 May 14. Nat Rev Endocrinol. 2013. PMID: 23669656 Review. - Progesterone Receptor Signaling in Uterine Myometrial Physiology and Preterm Birth.
Wu SP, DeMayo FJ. Wu SP, et al. Curr Top Dev Biol. 2017;125:171-190. doi: 10.1016/bs.ctdb.2017.03.001. Epub 2017 Apr 26. Curr Top Dev Biol. 2017. PMID: 28527571 Free PMC article. Review.
Cited by
- miR-200 Regulates Endometrial Development During Early Pregnancy.
Jimenez PT, Mainigi MA, Word RA, Kraus WL, Mendelson CR. Jimenez PT, et al. Mol Endocrinol. 2016 Sep;30(9):977-87. doi: 10.1210/me.2016-1050. Epub 2016 Aug 17. Mol Endocrinol. 2016. PMID: 27533790 Free PMC article. - miR-139-5p suppresses cancer cell migration and invasion through targeting ZEB1 and ZEB2 in GBM.
Yue S, Wang L, Zhang H, Min Y, Lou Y, Sun H, Jiang Y, Zhang W, Liang A, Guo Y, Chen P, Lv G, Wang L, Zong Q, Li Y. Yue S, et al. Tumour Biol. 2015 Sep;36(9):6741-9. doi: 10.1007/s13277-015-3372-8. Epub 2015 Apr 3. Tumour Biol. 2015. PMID: 25833697 - Differential expression of microRNAs in retinal vasculopathy caused by selective Müller cell disruption.
Chung SH, Gillies M, Yam M, Wang Y, Shen W. Chung SH, et al. Sci Rep. 2016 Jul 4;6:28993. doi: 10.1038/srep28993. Sci Rep. 2016. PMID: 27373709 Free PMC article. - Myometrial progesterone receptor determines a transcription program for uterine remodeling and contractions during pregnancy.
Wu SP, Wang T, Yao ZC, Peavey MC, Li X, Zhou L, Larina IV, DeMayo FJ. Wu SP, et al. PNAS Nexus. 2022 Aug 19;1(4):pgac155. doi: 10.1093/pnasnexus/pgac155. eCollection 2022 Sep. PNAS Nexus. 2022. PMID: 36120506 Free PMC article. - Heightened uterine mammalian target of rapamycin complex 1 (mTORC1) signaling provokes preterm birth in mice.
Hirota Y, Cha J, Yoshie M, Daikoku T, Dey SK. Hirota Y, et al. Proc Natl Acad Sci U S A. 2011 Nov 1;108(44):18073-8. doi: 10.1073/pnas.1108180108. Epub 2011 Oct 24. Proc Natl Acad Sci U S A. 2011. PMID: 22025690 Free PMC article.
References
- Mesiano S, et al. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. - PubMed
- Condon JC, Jeyasuria P, Faust JM, Wilson JW, Mendelson CR. A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. Proc Natl Acad Sci USA. 2003;100:9518–9523. - PMC - PubMed
- Bollapragada S, et al. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200(104):e1–e11. - PubMed
- Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem. 1996;271:6217–6224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials