Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics - PubMed (original) (raw)
Review
Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics
Cynthia X Ma et al. Trends Mol Med. 2011 Feb.
Abstract
Defects in p53 function, which occur frequently in human cancers due to mutations in TP53 or disruptions in the p53 regulatory pathway, render cells dependent on CHK1 (Checkpoint Kinase 1) to activate cell cycle checkpoints. In the presence of DNA damage or replication stress, inhibition of CHK1 leads to "mitotic catastrophe" and cell death in p53-deficient tumors while sparing p53-proficient cells. CHK1 inhibitors sensitize tumors to a variety of DNA-damaging agents or antimetabolites in preclinical models and are being evaluated in early phase clinical trials. In this review, we summarize recent advances and controversies in the development and application of CHK1 inhibitors as cancer therapeutics.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
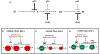
Figure 1.. Cell cycle checkpoints
The eukaryotic cell cycle consists of four phases called G1 (Gap 1), S (Synthesis), G2 (Gap 2) and M (Mitosis). Genotoxic and replicative stress activate checkpoints in order to delay cells from transitioning from one cell cycle phase to the next. p53 is required for cells to stop at the G1/S-border (G1 checkpoint) whereas CHK1 is required to prevent new replication origins from firing in S-phase (S-phase checkpoint) and to prevent cells from exiting G2- and entering into M-phase (G2 checkpoint). Although cells are able to activate the S- and G2- checkpoints in the absence of p53, they are unable to sustain these checkpoints for as long as normal cells (A, B). Most cancer cells lack a functional p53 pathway and therefore are unable to arrest in G1 when their DNA is damaged, but they are able to activate the S- and G2-checkpoints through the CHK1 pathway. This gives the tumor cells time to repair any DNA damage and this promotes their survival (C). When p53 deficient cancer cells are subjected to genotoxic or replicative stress in combination with a CHK1 inhibitor they lose all three checkpoints, and progress through the cell cycle without repairing their DNA damage. This results in preferential killing of p53-deficient tumor cells (D).
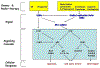
Figure 2.. DNA damage response pathway
Exposure of cells to IR or etoposide induces double strand breaks (DSBs) in DNA whereas exposure of cells to various chemotherapeutic agents (irinotecan, topotecan, cisplatin, carboplatin) or antimetabolites (gemcitabine, 5-fluorouracil, cytarabine) results in replication fork stalling and the generation of single strand breaks (SSBs). This, in turn, activates checkpoints that mobilize DNA repair pathways and either signals to the cell cycle machinery to prevent progression or induces apoptosis. ssDNA becomes coated with replication protein A (RPA), which recruits ATR as well as additional proteins thereby leading to full ATR activation. ATR phosphorylates many intracellular substrates including p53 and CHK1. ATR phosphorylates CHK1 on serines 317 and 345 resulting in CHK1 autophosphorylation on serine 296. Activated CHK1, in turn, phosphorylates the Cdc25A protein phosphatase to promote its ubiquitin-mediated proteolysis. Loss of Cdc25A results in cell cycle arrest in the S- and G2-phases of the cell division cycle. CHK1 also phosphorylates RAD51, FAND2 and FANCE to activate DNA repair pathways. DSBs activate ATM, which in turn phosphorylates both CHK2 and p53. p53 is also phosphorylated by CHK2. This leads to p53 accumulation and activation of its downstream target genes. Transcriptional activation of genes encoding BAX and PUMA lead to apoptosis whereas transcriptional activation of genes encoding p21 and 14–3-3s lead to G1 cell cycle arrest and also function to enforce the S- and G2-cell cycle arrests regulated by CHK1. Crosstalk exists between these pathways as stalled replication forks can lead to DSBs leading to ATM activation and the repair of DSBs can produce RPA-coated ssDNA that activates that ATR pathway [88].
Similar articles
- Selective Chk1 inhibitors differentially sensitize p53-deficient cancer cells to cancer therapeutics.
Chen Z, Xiao Z, Gu WZ, Xue J, Bui MH, Kovar P, Li G, Wang G, Tao ZF, Tong Y, Lin NH, Sham HL, Wang JY, Sowin TJ, Rosenberg SH, Zhang H. Chen Z, et al. Int J Cancer. 2006 Dec 15;119(12):2784-94. doi: 10.1002/ijc.22198. Int J Cancer. 2006. PMID: 17019715 - Characterization and preclinical development of LY2603618: a selective and potent Chk1 inhibitor.
King C, Diaz H, Barnard D, Barda D, Clawson D, Blosser W, Cox K, Guo S, Marshall M. King C, et al. Invest New Drugs. 2014 Apr;32(2):213-26. doi: 10.1007/s10637-013-0036-7. Epub 2013 Oct 10. Invest New Drugs. 2014. PMID: 24114124 - Chk1 inhibitors for novel cancer treatment.
Tao ZF, Lin NH. Tao ZF, et al. Anticancer Agents Med Chem. 2006 Jul;6(4):377-88. doi: 10.2174/187152006777698132. Anticancer Agents Med Chem. 2006. PMID: 16842237 Review. - Enhancement of hypoxia-activated prodrug TH-302 anti-tumor activity by Chk1 inhibition.
Meng F, Bhupathi D, Sun JD, Liu Q, Ahluwalia D, Wang Y, Matteucci MD, Hart CP. Meng F, et al. BMC Cancer. 2015 May 21;15:422. doi: 10.1186/s12885-015-1387-6. BMC Cancer. 2015. PMID: 25994202 Free PMC article. - G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer.
Bucher N, Britten CD. Bucher N, et al. Br J Cancer. 2008 Feb 12;98(3):523-8. doi: 10.1038/sj.bjc.6604208. Epub 2008 Jan 29. Br J Cancer. 2008. PMID: 18231106 Free PMC article. Review.
Cited by
- DNA damage response-related ncRNAs as regulators of therapy resistance in cancer.
Gao Z, Luan X, Wang X, Han T, Li X, Li Z, Li P, Zhou Z. Gao Z, et al. Front Pharmacol. 2024 Aug 26;15:1390300. doi: 10.3389/fphar.2024.1390300. eCollection 2024. Front Pharmacol. 2024. PMID: 39253383 Free PMC article. Review. - Triple-Negative Breast Cancer and Emerging Therapeutic Strategies: ATR and CHK1/2 as Promising Targets.
Sofianidi A, Dumbrava EE, Syrigos KN, Nasrazadani A. Sofianidi A, et al. Cancers (Basel). 2024 Mar 13;16(6):1139. doi: 10.3390/cancers16061139. Cancers (Basel). 2024. PMID: 38539474 Free PMC article. Review. - Prediction of immune infiltration and prognosis for patients with cholangiocarcinoma based on a cuproptosis-related lncRNA signature.
Yao HF, He M, Zhu YH, Zhang B, Chen PC, Huo YM, Zhang JF, Yang C. Yao HF, et al. Heliyon. 2023 Dec 20;10(1):e22774. doi: 10.1016/j.heliyon.2023.e22774. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226253 Free PMC article. - Small molecule targeting of the p38/Mk2 stress signaling pathways to improve cancer treatment.
Alimbetov D, Umbayev B, Tsoy A, Begimbetova D, Davis T, Kipling D, Askarova S. Alimbetov D, et al. BMC Cancer. 2023 Sep 23;23(1):895. doi: 10.1186/s12885-023-11319-x. BMC Cancer. 2023. PMID: 37740222 Free PMC article. - Novel Cellular Functions of ATR for Therapeutic Targeting: Embryogenesis to Tumorigenesis.
Biswas H, Makinwa Y, Zou Y. Biswas H, et al. Int J Mol Sci. 2023 Jul 20;24(14):11684. doi: 10.3390/ijms241411684. Int J Mol Sci. 2023. PMID: 37511442 Free PMC article. Review.
References
- Schlegel R, and Pardee AB (1986) Caffeine-induced uncoupling of mitosis from the completion of DNA replication in mammalian cells. Science 232, 1264–1266 - PubMed
- Fan S, et al. (1995) Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res 55, 1649–1654 - PubMed
- Powell SN, et al. (1995) Differential Sensitivity of p53(−) and p53(+) cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Research 55, 1643–1648 - PubMed
- Russell KJ, et al. (1995) Abrogation of the G2 checkpoint results in differential radiosensitization of G1 checkpoint-deficient and G1 checkpoint-competent cells. Cancer Res 55, 1639–1642 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous