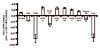Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis - PubMed (original) (raw)
Combination of Dll4/Notch and Ephrin-B2/EphB4 targeted therapy is highly effective in disrupting tumor angiogenesis
Dusan Djokovic et al. BMC Cancer. 2010.
Abstract
Background: Dll4/Notch and Ephrin-B2/EphB4 pathways play critical roles in tumor vessel development and maturation. This study evaluates the efficacy of the inhibition of both signaling pathways, alone and in combination, in reducing the growth of an autochthonous mouse tumor and assesses potential adverse effects.
Methods: We used the transgenic RIP1-Tag2 tumor model to study the effects of 1) inhibition of Dll4/Notch by either Dll4 allelic deletion or use of a soluble extracellular Dll4 (sDll4), 2) inhibition of Ephrin-B2/EphB4 signaling by a soluble extracellular EphB4 fused to albumin (sEphB4-Alb), and 3) inhibition of both pathways by sEphB4-Alb combined with either Dll4 allelic deletion or sDll4. To investigate adverse effects, we used inducible endothelial-specific Dll4 knock-out mice, treated with sEphB4-Alb, and carried out histopathological analysis.
Results: Dll4 allele deletion or soluble Dll4 treatment resulted in increased tumor vessel density, reduced mural cell recruitment and vessel perfusion which resulted in reduced tumor size. The soluble EphB4 instead reduced vessel density and vessel perfusion, leading to reduction of tumor size. Greater efficacy was observed when sEphB4-Alb was combined with either Dll4 allele deletion or sDll4 in regards to tumor size, vessel perfusion and mural cell recruitment. Induced endothelial specific Dll4 loss-of-function caused hepatic vascular alterations, which were prevented by concomitant sEphB4-Alb treatment.
Conclusion: Combination targeting of Dll4/Notch and Ephrin-B2/EphB4 has potential for clinical investigation, providing cumulative efficacy and increased safety over Dll4/Notch inhibition alone.
Figures
Figure 1
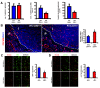
Dll4 allelic deletion reduced tumor burden in RT2 mice due to increased nonproductive tumor angiogenesis. A, Number of tumors per animal, average tumor volume and overall tumor burden, are calculated as the sum of tumor volumes per mouse, in RT2 Dll4+/+ (n = 8) and RT2 Dll4+/- (n = 8) mice. B, Vascular response examined in RT2 Dll4+/+ (n = 5) and RT2 Dll4+/- (n = 5) insulinomas by PECAM immunostaining. RT2 Dll4+/- mice showed reduced vessel calibers, increased sprouting, branching irregularities, and network disorganization (left). Dotted lines mark tumor border. I indicates insulinoma while P indicates normal pancreatic tissue surrounding the tumor. Vascular density was estimated as percentage of PECAM-positive area per tumor section surface and presented for two experimental groups (right). C, Mural cell coverage on newly formed vessels was examined by double staining of PECAM and α-SMA. SMA-positive cells lining PECAM-positive endothelium were profoundly reduced in RT2 Dll4+/- insulinomas (left). The percentage of PECAM-positive structures covered by SMA-positive area was measured as an indicator of vessel maturation (right). D, Tumor vessel competence evaluated by lectin perfusion. Simultaneous staining of endothelial cells with PECAM and visualization of perfused vessels demonstrate a reduction in the fraction of perfused vessels in RT2 Dll4+/- vs. RT2 Dll4+/+ mice (left). The percentage of PECAM-positive area co-localized with lectin was measured to estimate the proportion of functional vessels within insulinomas (right). The two group values for each parameter were statistically analyzed using Mann-Whitney-Wilcoxon test. Error bars represent SEM. * P < 0.05 was considered significant.
Figure 2
Differential gene expression in RT2 Dll4+/+ vs. RT2 Dll4+/- insulinomas. Total RNA was isolated from harvested insulinomas (2-3 tumors/mouse, n = 5 for each genotype) and gene expression analysis of tumor tissues was performed by quantitative RT-PCR for indicated genes involved in angiogenesis. Gene expression levels were normalized to β-actin levels. Error bars represent SD.
Figure 3
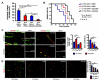
sEphB4-Alb inhibited insulinoma growth and extended longevity in RT2 Dll4+/+ and Dll4+/- mice. A, Average tumor volumes developed by 13.5-week old RT2 (Dll4+/+ or Dll4+/-) male mice previously treated for 3.5 weeks with drug vehicle (PBS) or sEphB4-Alb (10 mg/kg). All the groups had 6 animals and were treated i.p. 3 times a week. B, Survival rates in RT2 Dll4+/+ and RT2 Dll4+/- treated with vehicle (PBS) or sEphB4-Alb (5 mg/kg). All the groups had 10 animals and were treated i.p. 3 times a week. Survival rate was calculated as the percentage of live mice at the end of each week relative to the initial number of the animals per experimental group. C, Sections of tumors harvested at the end of the experiment mentioned in A were co-stained with anti-PECAM (red) and anti-α-SMA (green) as described in Fig. 1_C_. D, Sections of tumors harvested at the end of the experiment mentioned in A were co-stained with anti-PECAM (red) and anti-NG2 (green). The percentage of PECAM-positive structures covered by NG2-positive area was measured as a parameter of pericyte recruitment (right). Kaplan-Meier product-limit estimation with Breslow generalized Wilcoxon test was used for survival analyses. Other data were analyzed using Mann-Whitney-Wilcoxon test. Error bars represent SEM. * P < 0.05 and ** P < 0.01 were considered significant and highly significant, respectively.
Figure 4
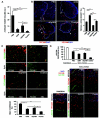
Combination therapy of sDll4-Fc and sEphB4Alb inhibits RT2 tumor growth with greater efficacy than either molecule alone. A, Average tumor volumes developed by 13.5-week old RT2 male mice previously treated for 3.5 weeks with drug vehicle (PBS, i.p. 3×/wk, control group), sEphB4-Alb (10 mg/kg i.p., 3×/wk), sDll4-Fc (10 mg/kg i.p. 3×/wk), or combination of sEphB4-Alb and sDll4-Fc (10 mg/kg, i.p. 3×/wk for both). The results were obtained from two independent trials involved 6 animals per treatment group and per trial. B, Sections of tumors harvested at the end of the experiment mentioned in A were immunostained with PECAM (red) and DAPI (blue) as described in Fig. 1_B_. C. Sections of tumors harvested at the end of the experiment mentioned in A were stained as described in Fig. 1_C_. D, Tumor vessel functionality evaluated by lectin perfusion as described in Fig. 1_D_. Perfusion in normal pancreas tissue was also analyzed and shown on the bottom left. The data were analyzed using Mann-Whitney-Wilcoxon test. Error bars represent SEM. * P < 0.05 and ** P < 0.01 were considered significant and highly significant, respectively.
Figure 5
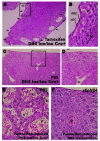
Hepatic vascular lesions observed in endothelial-specific Dll4 knock-out mice are prevented by sEphB4-Alb administration. Representative pictures of hematoxylin and eosin staining of liver sections from induced, tamoxifen-treated (A and B) and non-induced, PBS-treated (C and D) Dll4lox/lox Cre+ mice, and from induced, tamoxifen-treated Dll4lox/lox Cre+ mice administered with PBS vehicle (E) or sEphB4-Alb (F) for 12 weeks to assess the effect of Ephrin-B2/EphB4 blockade combined with total inhibition of Dll4/Notch endothelial signaling. A, Ten weeks after the induction of endothelium-specific Dll4 loss-of-function, all studied 14-week-old Dll4lox/lox Cre+ presented excessive sub-capsular vascular proliferation. The image shows vessels (arrows), some of which extremely dilated, occupying sub-capsular regions throughout the liver. B, Higher magnification of sub-capsular blood vessels (inset in A); C, Age-matched non-induced Dll4lox/lox Cre+ presented normal liver histology. D, Higher magnification of the inset in C showing a single hepatic vessel that cannot be considered sub-capsular. E and F, Excessive vascular proliferation forming an hemangioma-like sub-capsular structure in a tamoxifen-induced Dll4lox/lox Cre+ mouse injected with PBS and normal hepatic structure in a sEphB4-treated tamoxifen-induced Dll4lox/lox Cre+ mouse, respectively. V, blood vessel; nEC, endothelial cell nucleus, RBC, red blood cells; H, hepatocytes.
Similar articles
- Vascular endothelial growth factor-A inhibits EphB4 and stimulates delta-like ligand 4 expression in adult endothelial cells.
Yang C, Guo Y, Jadlowiec CC, Li X, Lv W, Model LS, Collins MJ, Kondo Y, Muto A, Shu C, Dardik A. Yang C, et al. J Surg Res. 2013 Jul;183(1):478-86. doi: 10.1016/j.jss.2013.01.009. Epub 2013 Feb 1. J Surg Res. 2013. PMID: 23394931 Free PMC article. - Endothelial Dll4 overexpression reduces vascular response and inhibits tumor growth and metastasization in vivo.
Trindade A, Djokovic D, Gigante J, Mendonça L, Duarte A. Trindade A, et al. BMC Cancer. 2017 Mar 14;17(1):189. doi: 10.1186/s12885-017-3171-2. BMC Cancer. 2017. PMID: 28288569 Free PMC article. - EphB4 forward signalling mediates angiogenesis caused by CCM3/PDCD10-ablation.
You C, Zhao K, Dammann P, Keyvani K, Kreitschmann-Andermahr I, Sure U, Zhu Y. You C, et al. J Cell Mol Med. 2017 Sep;21(9):1848-1858. doi: 10.1111/jcmm.13105. Epub 2017 Apr 1. J Cell Mol Med. 2017. PMID: 28371279 Free PMC article. - Molecular identity of arteries, veins, and lymphatics.
Wolf K, Hu H, Isaji T, Dardik A. Wolf K, et al. J Vasc Surg. 2019 Jan;69(1):253-262. doi: 10.1016/j.jvs.2018.06.195. Epub 2018 Aug 25. J Vasc Surg. 2019. PMID: 30154011 Free PMC article. Review. - The critical role of the interplays of EphrinB2/EphB4 and VEGF in the induction of angiogenesis.
Du E, Li X, He S, Li X, He S. Du E, et al. Mol Biol Rep. 2020 Jun;47(6):4681-4690. doi: 10.1007/s11033-020-05470-y. Epub 2020 Jun 2. Mol Biol Rep. 2020. PMID: 32488576 Review.
Cited by
- Low-dosage inhibition of Dll4 signaling promotes wound healing by inducing functional neo-angiogenesis.
Trindade A, Djokovic D, Gigante J, Badenes M, Pedrosa AR, Fernandes AC, Lopes-da-Costa L, Krasnoperov V, Liu R, Gill PS, Duarte A. Trindade A, et al. PLoS One. 2012;7(1):e29863. doi: 10.1371/journal.pone.0029863. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279550 Free PMC article. - Progress in tumor vascular normalization for anticancer therapy: challenges and perspectives.
Shang B, Cao Z, Zhou Q. Shang B, et al. Front Med. 2012 Mar;6(1):67-78. doi: 10.1007/s11684-012-0176-8. Epub 2012 Mar 31. Front Med. 2012. PMID: 22460450 Review. - The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment.
Papadakos SP, Dedes N, Gkolemi N, Machairas N, Theocharis S. Papadakos SP, et al. Int J Mol Sci. 2023 Feb 3;24(3):3015. doi: 10.3390/ijms24033015. Int J Mol Sci. 2023. PMID: 36769332 Free PMC article. Review. - The roles of metabolic profiles and intracellular signaling pathways of tumor microenvironment cells in angiogenesis of solid tumors.
Zalpoor H, Aziziyan F, Liaghat M, Bakhtiyari M, Akbari A, Nabi-Afjadi M, Forghaniesfidvajani R, Rezaei N. Zalpoor H, et al. Cell Commun Signal. 2022 Nov 23;20(1):186. doi: 10.1186/s12964-022-00951-y. Cell Commun Signal. 2022. PMID: 36419156 Free PMC article. Review. - Dll4-Notch signaling as a therapeutic target in tumor angiogenesis.
Kuhnert F, Kirshner JR, Thurston G. Kuhnert F, et al. Vasc Cell. 2011 Sep 18;3(1):20. doi: 10.1186/2045-824X-3-20. Vasc Cell. 2011. PMID: 21923938 Free PMC article.
References
- Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101(45):15949–54. doi: 10.1073/pnas.0407290101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous