Pelizaeus-Merzbacher-like disease caused by AIMP1/p43 homozygous mutation - PubMed (original) (raw)
. 2010 Dec 10;87(6):820-8.
doi: 10.1016/j.ajhg.2010.10.016. Epub 2010 Nov 18.
Barak Markus, Iris Noyman, Hannah Shalev, Hagit Flusser, Ilan Shelef, Keren Liani-Leibson, Zamir Shorer, Idan Cohen, Shareef Khateeb, Sara Sivan, Ohad S Birk
Affiliations
- PMID: 21092922
- PMCID: PMC2997381
- DOI: 10.1016/j.ajhg.2010.10.016
Pelizaeus-Merzbacher-like disease caused by AIMP1/p43 homozygous mutation
Miora Feinstein et al. Am J Hum Genet. 2010.
Abstract
Pelizaeus-Merzbacher disease is an X-linked hypomyelinating leukodystrophy caused by PLP1 mutations. A similar autosomal-recessive phenotype, Pelizaeus-Merzbacher-like disease (PMLD), has been shown to be caused by homozygous mutations in GJC2 or HSPD1. We report a consanguineous Israeli Bedouin kindred with clinical and radiological findings compatible with PMLD in which linkage to PLP1, GJC2, and HSPD1 was excluded. Through genome-wide homozygosity mapping and mutation analysis, we demonstrated in all affected individuals a homozygous frameshift mutation that fully abrogates the main active domain of AIMP1, encoding ARS-interacting multifunctional protein 1. The mutation fully segregates with the disease-associated phenotype and was not found in 250 Bedouin controls. Our findings are in line with the previously demonstrated inability of mutant mice lacking the AIMP1/p43 ortholog to maintain axon integrity in the central and peripheral neural system.
Copyright © 2010 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
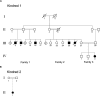
Pedigree of the Affected Israeli Bedouin Kindred (A and B) The pedigree is compatible with autosomal-recessive heredity. (A) and (B) are branches of a single extended tribe, remotely related (not first or second degree).
Figure 2
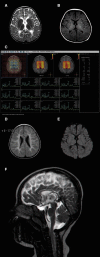
MRI of Two Affected Individuals (A–C) Girl age 5 years with severe form of PMLD. The images demonstrate arrested myelination and reduced N-acetylaspartate. The T2-weighted images showed high signal of hypomyelination starting at a normal stage (A), as well as high signal on flair (B). Magnetic resonance spectroscopy demonstrated relative decrease of N-acetylaspartate within the white matter, likely because of axonal degeneration (C). (D–F) Girl age 14 months with severe form of PMLD. The images demonstrate incomplete myelination and corpus callosum atrophy. These findings were seen as low signal on T1-weighted images (D). On diffusion-weighted images, eADC showed low signal (E) representing increased diffusivity secondary to hypomyelinated brain tissue. Generalized brain atrophy, especially of the corpus callosum, was noted (F).
Figure 3
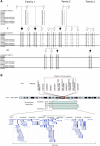
Fine Mapping of the 4q24 Locus in Kindred 1 and Kindred 2 (A) Disease-associated haplotype shown in boxes. Markers D4S2634 and chr4:107336226-107336269 define the minimal homozygosity locus associated with the disease. Blank spaces indicate nongenotyped markers. (B) Schematic presentation of the defined locus and the genes it contains (UCSC). Markers within the homozygosity locus are boxed.
Figure 4
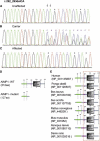
The c.292_293delCA Mutation in Exon 4 of AIMP1 (A–C) Sequence analysis is shown for an unaffected individual (A), an obligatory carrier (B), and an affected individual (C). (D) SMART display of human AIMP1 domains along the wild-type sequence versus the mutant protein. The aberrant region in the mutant protein includes the tRNA-binding domain, a sequence needed for interaction with HSP90B1, and a region required for endothelial cell death and migration. The protein sequence is altered after 97 aa, and a stop codon truncates the protein after 127 aa. (E) Conservation of AIMP1 domains throughout evolution. Deleted area is boxed.
Comment in
- AIMP1/p43 mutation and PMLD.
Biancheri R, Rossi A, Zara F, Filocamo M. Biancheri R, et al. Am J Hum Genet. 2011 Mar 11;88(3):391; author reply 393-5. doi: 10.1016/j.ajhg.2011.02.003. Am J Hum Genet. 2011. PMID: 21397066 Free PMC article. No abstract available. - Neurodegenerative disorder related to AIMP1/p43 mutation is not a PMLD.
Boespflug-Tanguy O, Aubourg P, Dorboz I, Bégou M, Giraud G, Sarret C, Vaurs-Barrière C. Boespflug-Tanguy O, et al. Am J Hum Genet. 2011 Mar 11;88(3):392-3; author reply 393-5. doi: 10.1016/j.ajhg.2010.12.015. Am J Hum Genet. 2011. PMID: 21397067 Free PMC article. No abstract available.
Similar articles
- Pathogenic variants in AIMP1 cause pontocerebellar hypoplasia.
Accogli A, Russell L, Sébire G, Rivière JB, St-Onge J, Addour-Boudrahem N, Laporte AD, Rouleau GA, Saint-Martin C, Srour M. Accogli A, et al. Neurogenetics. 2019 May;20(2):103-108. doi: 10.1007/s10048-019-00572-7. Epub 2019 Mar 28. Neurogenetics. 2019. PMID: 30924036 - AIMP1/p43 mutation and PMLD.
Biancheri R, Rossi A, Zara F, Filocamo M. Biancheri R, et al. Am J Hum Genet. 2011 Mar 11;88(3):391; author reply 393-5. doi: 10.1016/j.ajhg.2011.02.003. Am J Hum Genet. 2011. PMID: 21397066 Free PMC article. No abstract available. - Hypomyelinating Leukodystrophy due to HSPD1 Mutations: A New Patient.
Kusk MS, Damgaard B, Risom L, Hansen B, Ostergaard E. Kusk MS, et al. Neuropediatrics. 2016 Oct;47(5):332-5. doi: 10.1055/s-0036-1584564. Epub 2016 Jul 12. Neuropediatrics. 2016. PMID: 27405012 - Pelizaeus-Merzbacher disease, Pelizaeus-Merzbacher-like disease 1, and related hypomyelinating disorders.
Hobson GM, Garbern JY. Hobson GM, et al. Semin Neurol. 2012 Feb;32(1):62-7. doi: 10.1055/s-0032-1306388. Epub 2012 Mar 15. Semin Neurol. 2012. PMID: 22422208 Review. - Pelizaeus-Merzbacher Disease: Molecular and Cellular Pathologies and Associated Phenotypes.
Inoue K. Inoue K. Adv Exp Med Biol. 2019;1190:201-216. doi: 10.1007/978-981-32-9636-7_13. Adv Exp Med Biol. 2019. PMID: 31760646 Review.
Cited by
- Aminoacyl-tRNA Synthetase: A Non-Negligible Molecule in RNA Viral Infection.
Feng M, Zhang H. Feng M, et al. Viruses. 2022 Mar 15;14(3):613. doi: 10.3390/v14030613. Viruses. 2022. PMID: 35337020 Free PMC article. Review. - Rare Neurologic Disease-Associated Mutations of AIMP1 are Related with Inhibitory Neuronal Differentiation Which is Reversed by Ibuprofen.
Takeuchi Y, Tanaka M, Okura N, Fukui Y, Noguchi K, Hayashi Y, Torii T, Ooizumi H, Ohbuchi K, Mizoguchi K, Miyamoto Y, Yamauchi J. Takeuchi Y, et al. Medicines (Basel). 2020 May 6;7(5):25. doi: 10.3390/medicines7050025. Medicines (Basel). 2020. PMID: 32384815 Free PMC article. - Recessive mutations in POLR3B, encoding the second largest subunit of Pol III, cause a rare hypomyelinating leukodystrophy.
Tétreault M, Choquet K, Orcesi S, Tonduti D, Balottin U, Teichmann M, Fribourg S, Schiffmann R, Brais B, Vanderver A, Bernard G. Tétreault M, et al. Am J Hum Genet. 2011 Nov 11;89(5):652-5. doi: 10.1016/j.ajhg.2011.10.006. Epub 2011 Oct 27. Am J Hum Genet. 2011. PMID: 22036172 Free PMC article. - 3-Dimensional architecture of the human multi-tRNA synthetase complex.
Khan K, Baleanu-Gogonea C, Willard B, Gogonea V, Fox PL. Khan K, et al. Nucleic Acids Res. 2020 Sep 4;48(15):8740-8754. doi: 10.1093/nar/gkaa569. Nucleic Acids Res. 2020. PMID: 32644155 Free PMC article. - Exome Sequence Analysis Suggests that Genetic Burden Contributes to Phenotypic Variability and Complex Neuropathy.
Gonzaga-Jauregui C, Harel T, Gambin T, Kousi M, Griffin LB, Francescatto L, Ozes B, Karaca E, Jhangiani SN, Bainbridge MN, Lawson KS, Pehlivan D, Okamoto Y, Withers M, Mancias P, Slavotinek A, Reitnauer PJ, Goksungur MT, Shy M, Crawford TO, Koenig M, Willer J, Flores BN, Pediaditrakis I, Us O, Wiszniewski W, Parman Y, Antonellis A, Muzny DM; Baylor-Hopkins Center for Mendelian Genomics; Katsanis N, Battaloglu E, Boerwinkle E, Gibbs RA, Lupski JR. Gonzaga-Jauregui C, et al. Cell Rep. 2015 Aug 18;12(7):1169-83. doi: 10.1016/j.celrep.2015.07.023. Epub 2015 Aug 6. Cell Rep. 2015. PMID: 26257172 Free PMC article.
References
- van der Knaap M.S., Breiter S.N., Naidu S., Hart A.A., Valk J. Defining and categorizing leukoencephalopathies of unknown origin: MR imaging approach. Radiology. 1999;213:121–133. - PubMed
- Magen D., Georgopoulos C., Bross P., Ang D., Segev Y., Goldsher D., Nemirovski A., Shahar E., Ravid S., Luder A. Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am. J. Hum. Genet. 2008;83:30–42. - PMC - PubMed
- Uhlenberg B., Schuelke M., Rüschendorf F., Ruf N., Kaindl A.M., Henneke M., Thiele H., Stoltenburg-Didinger G., Aksu F., Topaloğlu H. Mutations in the gene encoding gap junction protein alpha 12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am. J. Hum. Genet. 2004;75:251–260. - PMC - PubMed
- Salviati L., Trevisson E., Baldoin M.C., Toldo I., Sartori S., Calderone M., Tenconi R., Laverda A. A novel deletion in the GJA12 gene causes Pelizaeus-Merzbacher-like disease. Neurogenetics. 2007;8:57–60. - PubMed
- Birnbaum R.Y., Zvulunov A., Hallel-Halevy D., Cagnano E., Finer G., Ofir R., Geiger D., Silberstein E., Feferman Y., Birk O.S. Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein. Nat. Genet. 2006;38:749–751. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous