Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes - PubMed (original) (raw)
. 2010 Nov 24;33(5):736-51.
doi: 10.1016/j.immuni.2010.10.017.
Arthur Mortha, Viet L Bui, Pedro P Hernandez, Elina A Kiss, Thomas Hoyler, Melanie Flach, Bertram Bengsch, Robert Thimme, Christoph Hölscher, Manfred Hönig, Ulrich Pannicke, Klaus Schwarz, Carl F Ware, Daniela Finke, Andreas Diefenbach
Affiliations
- PMID: 21093318
- PMCID: PMC3042726
- DOI: 10.1016/j.immuni.2010.10.017
Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes
Cedric Vonarbourg et al. Immunity. 2010.
Abstract
Whether the recently identified innate lymphocyte population coexpressing natural killer cell receptors (NKRs) and the nuclear receptor RORγt is part of the NK or lymphoid tissue inducer (LTi) cell lineage remains unclear. By using adoptive transfer of genetically tagged LTi-like cells, we demonstrate that NKR⁻RORγt(+) innate lymphocytes but not NK cells were direct progenitors to NKR(+)RORγt(+) cells in vivo. Genetic lineage tracing revealed that the differentiation of LTi-like cells was characterized by the stable upregulation of NKRs and a progressive loss of RORγt expression. Whereas interleukin-7 (IL-7) and intestinal microbiota stabilized RORγt expression within such NKR-LTi cells, IL-12 and IL-15 accelerated RORγt loss. RORγt(+) NKR-LTi cells produced IL-22, whereas RORγt⁻ NKR-LTi cells released IFN-γ and were potent inducers of colitis. Thus, the RORγt gradient in NKR-LTi cells serves as a tunable rheostat for their functional program. Our data also define a previously unappreciated role of RORγt⁻ NKR-LTi cells for the onset or maintenance of inflammatory bowel diseases.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
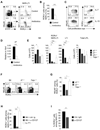
Figure 1. NKp46+RORγt+ cells are derived from NKp46−RORγt+ precursors
(A) Flow cytometry analysis of NKp46 and RORγt (EGFP) expression by CD3−CD19− lymphocytes from the indicated organs of _Rorc_gfp/+ mice. Numbers represent percent cells in quadrant. n.d.: not done. (B) 2×104 of the indicated cell populations from the small intestine of _Rorc_gfp/+ mice (H-2b) were transferred into _Rag2_−/−_Il2rg_−/− mice (H-2d). At the indicated timepoints, donor-derived lymphocytes were analyzed for NKp46 and RORγt (EGFP) expression. Numbers represent percent cells in quadrant. (C) Sorted cell populations from the intestinal lamina propria of _Rorc_gfp/+ mice were cultured with and without feeder cells for seven days in vitro. Contour plots show NKp46 and RORγt (EGFP) expression and were electronically gated on CD45+ cells. Numbers represent percent cells in quadrant. Data are representative of three (C) or four (A,B) independent experiments.
Figure 2. Genetic lineage tracing reveals progressive loss of RORγt in NKR-LTi cells
(A) Flow cytometry analysis of NKp46 and RORγt-fate map (EYFP) in CD3−CD19− lymphocytes from the indicated organs. Numbers represent percent cells in each quadrant. (B) Lymphocytes from the indicated organs of RORγt-fate map mice were stained with antibodies specific for CD3, CD19, NKp46 and RORγt or isotype control antibody. Histograms are electronically gated on CD3−CD19− cells and represent staining of the indicated cell populations with RORγt (grey) or isotype control Ab (open). Numbers indicate percentages of RORγt+ cells. (C) Percentage (± SEM, n=7) of RORγt+ cells among all NKR-LTi cells in the indicated organs. (D) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46 and CD4. Density plots are electronically gated on CD3−CD19− cells and depict the proportion of CD4 and NKp46 expressing cells within the indicated cell populations. (E) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46, CD4 and RORγt (grey) or with isotype control antibody (open). Histograms are gated on CD3− CD19− cells and depict expression of RORγt within CD4+ and CD4− NKR-LTi cells. Numbers indicate percentages of RORγt+ cells. (F) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46, CD25, CCR6 and RORγt. Density plotes are electronically gated on CD3−CD19− cells and show co-expression of RORγt and CD25 (top) or CCR6 (bottom). (G) 2×104 splenic CD4+RORγt+ NKR-LTi cells of _Rorc_gfp/+ mice (H-2b) were transferred into _Rag2_−/−_Il2rg_−/− mice (H-2d). Two weeks later, CD4 and RORγt (EGFP) expression by donor-derived cells (H-2b) was determined. Numbers represent percent cells in quadrants. (H) Splenocytes from CD4-fate map mice were stained for CD3, CD19, NKp46 and CD4. Contour plots are gated on CD3−CD19− cells and depict expression of NKp46 and CD4 or CD4-fate map (EYFP). Histogram shows the percentage of NKp46+CD4fm+ cells expressing CD4. Data are representative of ten (A), seven (C), four experiments (B, D–G) or two experiments (H).
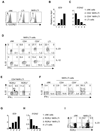
Figure 3. Commensal microflora and IL-7 stabilize RORγt expression in NKR-LTi cells
(A,B) Lymphocytes from the small intestine of conventional and antibiotic-treated RORγt-fate map mice were stained with antibodies specific for CD3, CD19, NKp46, NK1.1 and RORγt. Contour plots (left) are electronically gated on CD3−CD19− cells and depict expression of RORγtfm (EYFP) and NKp46. Dot plots (right) are electronically gated on NKR-LTi cells and represent staining for RORγt and NK1.1. Numbers indicate percentages. (B) Percentage (± SEM, n=3) of RORγt+ cells among NKR-LTi cells in conventional and antibiotic-treated RORγt-fate map mice. (C) Highly purified RORγt+ NKR-LTi cells from the small intestine were labelled with a cell proliferation dye and cultured in IL-7 or IL-15 for six days. Cells were stained for NKp46 and RORγt. Numbers indicate percentages in each quadrant. (D) Quantitative RT-PCR analysis of Il7 expression in small intestine of control mice and germ-free mice. (E) Absolute cell numbers (± SD, n=5) of the indicated cell populations in the lamina propria of the small intestine. (F,G) Lymphocytes of the small intestine were stained with antibodies specific for CD3, CD19, NKp46 and RORγt. Contour plots are electronically gated on CD3−CD19− cells and numbers indicate percentages within quadrants (F). (G) Percentage (± SD, n=5) of NKp46+ cells among all RORγt+ cells. (H) 2×104 highly purified RORγt+ NKR-LTi cells (CD45.1) from the small intestine were transferred into groups of B6 (CD45.2) or IL-7Tg mice (CD45.2). B6 mice were treated with IL-7Rα antibodies or control Ig. Percentages (± SEM, n=3) of RORγt+ cells among donor-derived cells (CD45.1+) were determined two weeks after transfer. (I) RORγt-fate map mice were treated with either IL-7Rα antibodies or control Ig. Percentages (± SEM, n=3) of RORγt+ cells among NKR-LTi cells were determined after six days. Data are representative of at least three independent experiments.
Figure 4. Human IL-22-producing cells depend on IL-7 for their development
(A,B) Quantitative RT-PCR analysis of IL22 and RORC expression by the indicated, populations from human tonsils (A) or peripheral blood (B). Stage 1: CD34+CD117− CD94−, Stage 2: CD34+CD117+CD94−, Stage 3: CD34−CD117+CD94−, Stage 4: CD34−CD117+CD94+. (C,D) Mononuclear cells were stained with antibodies specific for the indicated markers and for CD3 and CD19. Dot plots (C) were electronically gated on CD3−CD19− cells. Contour plots (D) exclude all CD3+ and CD19+ cells and are electronically gated on CD34+ (stage 1 and stage 2) or CD34− cells (stage 3 and stage 4). Numbers indicate percentage of cells within the adjacent gates. Data are representative of two independent experiments.
Figure 5. RORγt expression within NKR-LTi cells determines distinct functional fates
(A) Splenocytes from RORγt-fate map mice were stained for CD3, CD19, NKp46, IL-12/23Rβ1 (grey) or isotype control antibody (open). Histograms depict electronic gating on the indicated cell populations after exclusion of CD3+ and CD19+ cells. (B,C,G,H) Quantitative RT-PCR analysis of the expression of Il23r (B,G) and Il12rb2 (C,H) in sorted cell populations from spleen (B,C) or colon (G,H) of RORγt-fate map mice. (D,E) Cytokine production of highly purified cell populations from spleens of RORγt-fate map mice (D) or _Rorc_gfp/+ mice (E) stimulated for 24 hours with the indicated cytokines. Contour plots depict staining with anti-IL-22 and anti-IFN-γ. The numbers indicate the percentage of cells in each quadrant. (F) Groups of RORγt-fate map mice were injected with LPS. 18 hours later, splenocyte suspensions were stained with antibodies specific for CD3, CD19, NKp46, CD4, IL-22 and IFN-γ. Contour plots are electronically gated on the indicated cell populations after exclusion of all CD3+ and CD19+ cells. Numbers indicate percentage of cells in each quadrant. (I) Highly purified lamina propria lymphocytes from the colon of RORγt-fate map mice were cultured for 24h in IL-23. Contour plots depict expression of IFN-γ and RORγt. Numbers indicate the percentage of cells in each quadrant.
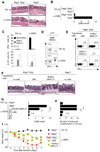
Figure 6. RORγt− NKR-LTi cells induce CD40-triggered colitis
(A–D) Groups of _Rag2_−/−, _Il15_−/− and _Rag2_−/−_Il2rg_−/− mice were injected with control Ig or anti-CD40 and were analyzed seven days later. (A) Histological analysis of colon sections (H&E stain). Arrowhead points to a cluster of infiltrating leukocytes that can only be found in mice with colitis. Bar = 100µm. (B) Clinical colitis score (± SEM; n=5). (C,D) Lamina propria lymphocytes from colon were stained for CD45, NKp46, RORγt, IL-22 and IFN-γ. (C) Bar diagrams show percentages (± SEM; n=5) of IFN-γ or IL-22-producing cells within the indicated lymphocyte populations. (D) Dot plots depict expression of IFN-γ and IL-22 by NKR+RORγt− cells. Numbers indicate the percentage of cells in each quadrant. (E–I) 5×104 highly purified cells from the intestine of RORγt-fate map mice (H-2b) were transferred into _Rag2_−/−_Il2rg_−/− mice (H-2d). (E) Five days after transfer, mice were injected with anti-CD40. Seven days after CD40 injection, donor cells were analyzed for IFN-γ production. Numbers indicate percentage of IFN-γ+ cells. (F–I) Two weeks after transfer mice were injected with control Ig or anti-CD40 and anlyzed seven days later. (F) Histological analysis of colon sections (H&E stain). Arrowheads point to clusters of infiltrating leukocytes that can only be found in mice with colitis. Bar = 100µm. (G) Clinical colitis score (± SEM; n=3). (H) Absolute numbers (± SEM; n=3) of leukocyte clusters (> 120 µm) per colon. (I) Weight as a percentage of the initial weight at day 0. Data represents the mean weight and is pooled from three independent experiments (n=5 mice per group). Error bars were omitted for clarity. Data represent the results of three independent experiments.
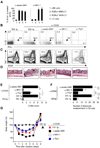
Figure 7. Depletion of RORγt− NKR-LTi cells ameliorates colitis
(A) Groups of RORγt-fate map were treated twice with the indicated antibodies. Two days after the last injection, intestinal lamina propria cells were stained with NKp46, RORγt, CD3 and CD19. After exclusion of all CD3+ and CD19+ cells, the indicated populations were quantified. The data represents the percentage (± SD; n=5) of remaining cells compared to control Ig-treated groups. (B–G) Groups of _Rag2_−/− mice were treated with the indicated antibodies and injected with control Ig or anti-CD40. Seven days later, lamina propria lymphocytes from colon were stained for CD45, NKp46, RORγt, IL-22, IFN-γ or TNF. The dot plots (B) represent the analysis of CD45+NKp46+RORγt− cells that contain both cNK cells and RORγt− NKR-LTi cells. Numbers represent percentage of cells in the respective quadrants. The contour plots (C) represent the analysis of all CD45+ cells. Numbers next to the areas indicate percentage of TNF+ cells within gates. (D) H&E staining of sections from the distal colon. Arrowheads point to inflammatory foci. Bar = 100 µm. (E) Clinical colitis score (± SEM, n=3). (F) Mean numbers (±SEM, n=3) of leukocyte clusters (> 120 µm) per colon. (G) Weight as a percentage of the initial weight at day 0. Data represents the mean weight and is pooled from three independent experiments (n=5 mice per group). Error bars were omitted for clarity.
Comment in
- The LTi cell, an immunologic chameleon.
Strober W. Strober W. Immunity. 2010 Nov 24;33(5):650-2. doi: 10.1016/j.immuni.2010.11.016. Immunity. 2010. PMID: 21094460 Free PMC article.
Similar articles
- Control of epithelial cell function by interleukin-22-producing RORγt+ innate lymphoid cells.
Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Sanos SL, et al. Immunology. 2011 Apr;132(4):453-65. doi: 10.1111/j.1365-2567.2011.03410.x. Immunology. 2011. PMID: 21391996 Free PMC article. Review. - IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors.
Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, Di Santo JP. Satoh-Takayama N, et al. J Exp Med. 2010 Feb 15;207(2):273-80. doi: 10.1084/jem.20092029. Epub 2010 Feb 8. J Exp Med. 2010. PMID: 20142427 Free PMC article. - Natural killer cell receptor-expressing innate lymphocytes: more than just NK cells.
Mortha A, Diefenbach A. Mortha A, et al. Cell Mol Life Sci. 2011 Nov;68(21):3541-55. doi: 10.1007/s00018-011-0803-6. Epub 2011 Sep 9. Cell Mol Life Sci. 2011. PMID: 21904914 Free PMC article. Review. - A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells.
Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d'Hargues Y, Göppert N, Croxford AL, Waisman A, Tanriver Y, Diefenbach A. Klose CS, et al. Nature. 2013 Feb 14;494(7436):261-5. doi: 10.1038/nature11813. Epub 2013 Jan 16. Nature. 2013. PMID: 23334414 - Differential Expression of the Transcription Factor GATA3 Specifies Lineage and Functions of Innate Lymphoid Cells.
Zhong C, Zheng M, Cui K, Martins AJ, Hu G, Li D, Tessarollo L, Kozlov S, Keller JR, Tsang JS, Zhao K, Zhu J. Zhong C, et al. Immunity. 2020 Jan 14;52(1):83-95.e4. doi: 10.1016/j.immuni.2019.12.001. Epub 2019 Dec 24. Immunity. 2020. PMID: 31882362 Free PMC article.
Cited by
- Interleukin 7 and thymic stromal lymphopoietin: from immunity to leukemia.
Tal N, Shochat C, Geron I, Bercovich D, Izraeli S. Tal N, et al. Cell Mol Life Sci. 2014 Feb;71(3):365-78. doi: 10.1007/s00018-013-1337-x. Epub 2013 Apr 27. Cell Mol Life Sci. 2014. PMID: 23625073 Free PMC article. Review. - Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis.
Perry JS, Han S, Xu Q, Herman ML, Kennedy LB, Csako G, Bielekova B. Perry JS, et al. Sci Transl Med. 2012 Aug 1;4(145):145ra106. doi: 10.1126/scitranslmed.3004140. Sci Transl Med. 2012. PMID: 22855463 Free PMC article. - Spectral Cytometry Has Unique Properties Allowing Multicolor Analysis of Cell Suspensions Isolated from Solid Tissues.
Schmutz S, Valente M, Cumano A, Novault S. Schmutz S, et al. PLoS One. 2016 Aug 8;11(8):e0159961. doi: 10.1371/journal.pone.0159961. eCollection 2016. PLoS One. 2016. PMID: 27500930 Free PMC article. - Critical Roles of Balanced Innate Lymphoid Cell Subsets in Intestinal Homeostasis, Chronic Inflammation, and Cancer.
Wu J, Lv X, Zhu S, Li T, Cheng H, Chen J. Wu J, et al. J Immunol Res. 2019 Nov 5;2019:1325181. doi: 10.1155/2019/1325181. eCollection 2019. J Immunol Res. 2019. PMID: 31781671 Free PMC article. Review. - Embryonic ILC-poiesis across tissues.
Hernández-Torres DC, Stehle C. Hernández-Torres DC, et al. Front Immunol. 2022 Dec 20;13:1040624. doi: 10.3389/fimmu.2022.1040624. eCollection 2022. Front Immunol. 2022. PMID: 36605193 Free PMC article. Review.
References
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. - PubMed
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37AI033068/AI/NIAID NIH HHS/United States
- R21 AI088445/AI/NIAID NIH HHS/United States
- R21 AI088445-01/AI/NIAID NIH HHS/United States
- R37 AI033068-20/AI/NIAID NIH HHS/United States
- R01 AI033068-09/AI/NIAID NIH HHS/United States
- CA069381/CA/NCI NIH HHS/United States
- R21 AI088445-02/AI/NIAID NIH HHS/United States
- R21 AI088445-03/AI/NIAID NIH HHS/United States
- R37 AI033068/AI/NIAID NIH HHS/United States
- R01 AI033068/AI/NIAID NIH HHS/United States
- P01 CA069381/CA/NCI NIH HHS/United States
- R01 AI033068-10/AI/NIAID NIH HHS/United States
- AI088445/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous