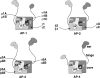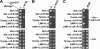Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants - PubMed (original) (raw)
Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants
Rafael Mattera et al. J Biol Chem. 2011.
Abstract
The clathrin-associated, heterotetrameric adaptor protein (AP) complexes, AP-1, AP-2, and AP-3, recognize signals in the cytosolic domains of transmembrane proteins, leading to their sorting to endosomes, lysosomes, lysosome-related organelles, and/or the basolateral membrane of polarized epithelial cells. One type of signal, referred to as "dileucine-based," fits the consensus motif (D/E)XXXL(L/I). Previous biochemical analyses showed that (D/E)XXXL(L/I) signals bind to a combination of two subunits of each AP complex, namely the AP-1 γ-σ1, AP-2 α-σ2, and AP-3 δ-σ3 hemicomplexes, and structural studies revealed that an imperfect variant of this motif lacking the (D/E) residue binds to a site straddling the interface of α and σ2. Herein, we report mutational and binding analyses showing that canonical (D/E)XXXL(L/I) signals bind to this same site on AP-2, and to similar sites on AP-1 and AP-3. The strength and amino acid requirements of different interactions depend on the specific signals and AP complexes involved. We also demonstrate the occurrence of diverse AP-1 heterotetramers by combinatorial assembly of various γ and σ1 subunit isoforms encoded by different genes. These AP-1 variants bind (D/E)XXXL(L/I) signals with marked preferences for certain sequences, implying that they are not functionally equivalent. Our results thus demonstrate that different AP complexes share a conserved binding site for (D/E)XXXL(L/I) signals. However, the characteristics of the binding site on each complex vary, providing for the specific recognition of a diverse repertoire of (D/E)XXXL(L/I) signals.
Figures
FIGURE 1.
Subunit heterogeneity of heterotetrameric AP complexes. The schematic shows the subunit composition and isoforms of the four AP complexes (for review, see Ref. 1). Combinatorial assembly of the various subunit isoforms could result in up to twelve AP-1 complexes, four AP-2, eight AP-3, and one AP-4. The inclusion of AP-1 β1 as an AP-2 subunit is based on the observed formation of β1-containing AP-2 complexes upon knockdown of AP-2 β2 (5) or disruption of the corresponding gene (6). The AP complexes have been represented according to the structures of the AP-1 and AP-2 core complexes (47, 50) and of the ear domains of AP-1 γ (51, 52), AP-2 α (–54) and AP-2 β2 (55). The schematic depicts a core comprising the trunk domains of the large subunits (γ, α, δ, or ϵ and β1-β4 for AP-1, -2, -3, or -4, respectively) together with the corresponding medium (μ) and small subunits (σ). The hinge and ear domains of the large subunits are shown protruding from the core of the complexes (see the AP-4 schematic). The depiction of two subdomains (an N-terminal IgG-like β sandwich and a C-terminal platform) in the ear domains of AP-1 β1, AP-3 δ, AP-3 β3, and AP-4 ϵ is based on alignment with AP-2 α and β2 subunits and secondary structure predictions. The prediction of a single C-terminal platform in AP-4 β4 is based on the lack of conservation of the N-terminal IgG-like β sandwich in this subunit as compared with the corresponding subunits in other AP complexes.
FIGURE 2.
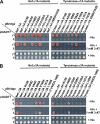
Y3H analysis of the interaction of (D/E)_XXX_L(L/I) signals with AP hemicomplexes. A, the schematic shows the two vectors used in the Y3H analysis. The cDNAs encoding the (D/E)_XXX_L(L/I) signals (full-length HIV-1Nef NL4–3 variant or the cytosolic tails of mouse tyrosinase or human LIMP-II) were subcloned into multiple cloning site 1 (MCS1) of the GAL4 binding domain vector pBridge, whereas the small AP subunits (σ1, σ2, σ3, or σ4) were subcloned into MCS2 of the same vector. The sequences encoding full-length mouse γ1, human γ2, rat αC, human δ, or human ϵ were subcloned into the GAL4 activation domain vector pGADT7. ori, origin of replication; Amp, ampicillin resistance gene; NLS, nuclear localization signal. B, sequences of the HIV-1 Nef (NL4–3 variant) flexible loop and mouse tyrosinase and human LIMP-II cytosolic tails with signals conforming to the (D/E)_XXX_L(L/I) are underlined. The diaspartate motif at +10 and +11 from the HIV-1 Nef ENTSLL dileucine signal that is required for binding to AP-2 (35) is also underlined. The mouse tyrosinase cytosolic tail contains a second (D/E)_XXX_L(L/I) motif (DYHSLL) C-terminal to the ERQPLL shown in the schematic. Although this second sequence is present in mouse and rat tyrosinase, it is not conserved in other species such as humans, is not involved in lysosomal/melanosomal sorting (56), and is not required for interaction with AP complexes (34). C, all (D/E)_XXX_L(L/I) signals tested interact with AP-1, AP-2, and AP-3 but not AP-4. Double transformants were plated in medium lacking histidine, leucine, tryptophan, and methionine (−His), to detect interaction among constructs, and in medium lacking only leucine, tryptophan, and methionine (+His) as a control for loading and viability of double transformants. In this experimental set, the −His plates were supplemented with a low concentration (0.2 m
m
) of 3-AT (a competitive inhibitor of the His3 protein) to minimize background growth due to nonspecific interactions. The lack of interaction of AP-4 with the various (D/E)_XXX_L(L/I) signals was also observed in −His plates lacking 3-AT. The image shown represents a composite of different plates from the same experiment. Results shown are representative of at least three experiments with similar results. Tyrase, tyrosinase. For details, see “Experimental Procedures.”
FIGURE 3.
Analysis of AP-2 residues involved in the interaction with Nef and tyrosinase (D/E)_XXX_L(L/I) signals. A, residues in the α (Arg21) and σ2 (Ala63, Val88, Asn92, Glu100, Leu101, and Leu103) subunits that were subjected to mutagenesis are shown in black on the surface representation of the three-dimensional structure of the AP-2 core complex (Protein Data Bank codes 1GW5 and 2VGL) (50). The α, β2, μ2, and σ2 subunits are depicted in light blue, green, magenta, and gold, respectively. Shown in red on the α subunit are residues Lys297 and Arg340, which are also required for the binding of the AP-2 α-σ2 hemicomplex to HIV-1 Nef (27). B, Y3H assays showing the effect of σ2 substitutions on the interaction of the AP-2 α-σ2 hemicomplex with HIV-1 Nef and tyrosinase (Tyrase) (D/E)_XXX_L(L/I) signals. Experiments were performed as indicated in the legend to Fig. 2. Positive controls included the interaction of (D/E)_XXX_L(L/I) signals with the AP-1 γ-σ1 and AP-2 α-σ2 hemicomplexes, whereas double transformants expressing (D/E)_XXX_L(L/I) signals and discordant γ and σ2 pairs were used as negative controls. Double transformants were plated on −His and −His plus 1 m
m
3-AT medium to analyze the interactions at different levels of stringency and on +His medium as a control for loading and viability. The image shown represents a composite of different plates from the same experiment.
FIGURE 4.
Conservation of AP-1 and AP-3 residues potentially involved in interactions with (D/E)_XXX_L(L/I) signals. A, alignment of human σ1A (RefSeq accession no. NP_001274), σ2 (GenBankTM accession no. AAH06337), and σ3A (GenBankTM accession no. CAG29337). The alignment shows the conservation of σ2 residues (Arg15, Ala63, Val88, Asn92, Glu100, Leu101, and Leu103 shown in black boxes) that interact with residues at different positions of the CD4 Q-peptide (20). Shown in black lettering are the positions on the signal (residue at −4; LL, dileucine pair; or O, other) proposed to bind to the σ residues in the corresponding boxes. B, alignment of N-terminal sequences of human γ1 (GenBankTM accession no. AAH36283), αC (Swiss-Prot accession no. O94973) and δ (GenBankTM accession number AAC51761) showing the conservation of the α subunit Arg residue (αArg15, shown in the black box) proposed to stabilize the −4 position of the CD4 Q-peptide (20). Alignments were generated with CLC Sequence Viewer; decreasing conservation of residues is shown by red to blue rainbow coloring.
FIGURE 5.
Mapping of AP-1 and AP-3 residues involved in interactions with (D/E)_XXX_L(L/I) signals. The interaction of (D/E)_XXX_L(L/I) motif-based signals with AP-1 γ-σ1 or AP-3 δ-σ3 mutant hemicomplexes is shown in A and B, respectively. Subcloning of cDNAs sequences encoding (D/E)_XXX_L(L/I) signals and AP subunits and plating of double transformants were performed as indicated in the legend to Fig. 2. Positive controls included the interaction of (D/E)_XXX_L(L/I) signals with the AP-1 γ-σ1 or AP-3 δ-σ3 hemicomplexes, whereas double transformants expressing (D/E)_XXX_L(L/I) signals and discordant γ-σ3 or δ-σ1 pairs were used as negative controls. The image shown represents a composite of different plates from the same experiment. Tyrase, tyrosinase.
FIGURE 6.
Substitution of γ, α, δ, and σ residues potentially interacting with the −4 position of the (D/E)_XXX_L(L/I) motif affects the interaction with HIV-Nef, tyrosinase, and LIMP-II signals. The interaction of the different (D/E)_XXX_L(L/I) signals with the wild-type or mutant γ-σ1, α-σ2, and δ-σ3 hemicomplexes is shown in A–C, respectively. Subcloning of cDNAs sequences encoding (D/E)_XXX_L(L/I) signals and AP subunits was performed as indicated in the legend to Fig. 2. Double transformants were plated on −His, −His plus 0.2 m
m
3-AT, −His plus 1 m
m
3-AT and +His medium (only −His plus 1 m
m
3-AT and +His plates shown for simplicity). Similar conclusions were drawn from the analysis of −His and −His plus 0.2 m
m
3-AT plates. Tyrase, tyrosinase.
FIGURE 7.
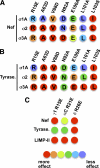
Graphic summary of the effects of substitutions in AP subunits on the interaction of (D/E)_XXX_L(L/I) motif-based signals. A and B, relative effect of substitutions in σ1A, σ2, and σ3 on the interaction with HIV-1 Nef (A) and tyrosinase signals (B) based on the results shown in Figs. 3 and 5. Numbering of residues indicated on top of schematics correspond to the σ2 sequence (see Figs. 4 and 5 for numbering of corresponding positions in σ1A and σ3A). The effect of substitutions is depicted ranging from red (binding completely abolished) to violet (no effect) in a rainbow gradient (see relative color gradient below C). A “blue shift” (lower sensitivity to mutations) can be observed for σ1A when comparing the effects of substitutions on this subunit with those at equivalent positions in σ2 or σ3A. C, relative effect of γ R15E, αC R21E, and δ R26E substitutions on the interaction with HIV-1 Nef, tyrosinase, and LIMP-II signals based on the results in Fig. 6. The effect of substitutions is depicted as indicated for A and B. Tyrase, tyrosinase.
FIGURE 8.
In vivo assembly of multiple AP-1 complexes containing different γ or σ1 subunit isoforms. A–D, M1 human fibroblasts were stably transfected with vectors driving the expression of σ1A-HA, σ1B-HA, σ1C-HA, or HA-γ2. Cells were metabolically labeled for 12–15 h with [35S]methionine and [35S]cysteine. Cell lysates were subjected to immunoprecipitation (IP) with anti-HA, and the immunoprecipitates were denatured, diluted, and subjected to additional rounds of immunoprecipitation (RC, recapture) using antibodies against the HA epitope, or μ1, β1, γ1, α, β3, or ϵ subunits (A–C) or σ1A, μ1, β1, HA, γ1, α, β3, or ϵ subunits (D). The recaptured immunoprecipitates were subjected to SDS-PAGE and fluorography. The analysis demonstrates that σ1A, σ1B, and σ1C subunits are all incorporated into AP-1 complexes also containing μ1, β1, and γ1 subunits, but not into AP-2, AP-3, or AP-4 (lack of recapture of σ1A, σ1B, or σ1C by either anti-α, -β3, or -ϵ) (A–C). The γ2 subunit also assembles into AP-1 but not AP-2, AP-3, or AP-4 (D). E, M1 cells stably transfected with HA-γ2 were subjected to transient transfection with vectors driving expression of σ1A-Myc, σ1B-Myc, or σ1C-Myc (a Myc-tagged stonin 2 proline-rich domain (Stn2 PRD) was used as a negative control). Transfected cells were metabolically labeled and lysed, followed by immunoprecipitation with anti-Myc and recapture with either anti-γ1 or anti-HA (for detection of γ2). Note that γ1 is incorporated into AP-1 complexes containing any of the three isoforms of σ1, whereas γ2 can only assemble into AP-1 complexes containing either σ1A or σ1B.
FIGURE 9.
γ subunit- and motif-dependent interaction of AP-1 with (D/E)_XXX_L(L/I) signals. All γ1-based AP-1 hemicomplexes (γ1-σ1A, γ1-σ1B, and γ1-σ1C) display similarly high avidities for (D/E)_XXX_L(L/I) signals. In contrast, γ2-σ1A and γ2-σ1B interact weakly with HIV-1 Nef, interact strongly with the tyrosinase tail, and do not recognize the LIMP-II signal. The lack of interaction detected for all double transformants expressing γ2 and σ1C is consistent with lack of assembly of AP-1 complexes comprising these two subunits, as evidenced by immunoprecipitation-recapture analysis of metabolically labeled cells (Fig. 8). This lack of interaction cannot be explained by lack of expression of γ2 and σ1C subunits in yeast given the binding of the tyrosinase signal to γ2-σ1A or γ2-σ1B and to γ1-σ1C, respectively. The image shown represents a composite of different plates from the same experiment. SV40 L T-Ag, SV40 large T-antigen (negative control for interactions with pBridge constructs and positive control for interaction with p53).
Similar articles
- The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site.
Doray B, Lee I, Knisely J, Bu G, Kornfeld S. Doray B, et al. Mol Biol Cell. 2007 May;18(5):1887-96. doi: 10.1091/mbc.e07-01-0012. Epub 2007 Mar 14. Mol Biol Cell. 2007. PMID: 17360967 Free PMC article. - Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes.
Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Janvier K, et al. J Cell Biol. 2003 Dec 22;163(6):1281-90. doi: 10.1083/jcb.200307157. J Cell Biol. 2003. PMID: 14691137 Free PMC article. - Signals for sorting of transmembrane proteins to endosomes and lysosomes.
Bonifacino JS, Traub LM. Bonifacino JS, et al. Annu Rev Biochem. 2003;72:395-447. doi: 10.1146/annurev.biochem.72.121801.161800. Epub 2003 Mar 6. Annu Rev Biochem. 2003. PMID: 12651740 Review. - Identification of acidic dileucine signals in LRP9 that interact with both GGAs and AP-1/AP-2.
Doray B, Knisely JM, Wartman L, Bu G, Kornfeld S. Doray B, et al. Traffic. 2008 Sep;9(9):1551-62. doi: 10.1111/j.1600-0854.2008.00786.x. Epub 2008 Jul 9. Traffic. 2008. PMID: 18627575 Free PMC article. - Adaptor proteins involved in polarized sorting.
Bonifacino JS. Bonifacino JS. J Cell Biol. 2014 Jan 6;204(1):7-17. doi: 10.1083/jcb.201310021. J Cell Biol. 2014. PMID: 24395635 Free PMC article. Review.
Cited by
- The clathrin adaptor complexes as a paradigm for membrane-associated allostery.
Canagarajah BJ, Ren X, Bonifacino JS, Hurley JH. Canagarajah BJ, et al. Protein Sci. 2013 May;22(5):517-29. doi: 10.1002/pro.2235. Epub 2013 Mar 18. Protein Sci. 2013. PMID: 23424177 Free PMC article. Review. - Intracellular nucleic acid sensors and autoimmunity.
Theofilopoulos AN, Kono DH, Beutler B, Baccala R. Theofilopoulos AN, et al. J Interferon Cytokine Res. 2011 Dec;31(12):867-86. doi: 10.1089/jir.2011.0092. Epub 2011 Oct 27. J Interferon Cytokine Res. 2011. PMID: 22029446 Free PMC article. Review. - AP-3 adaptor complex-mediated vesicle trafficking.
Ma Z, Islam MN, Xu T, Song E. Ma Z, et al. Biophys Rep. 2021 Apr 30;7(2):91-100. doi: 10.52601/bpr.2021.200051. Biophys Rep. 2021. PMID: 37288146 Free PMC article. - Clathrin-associated AP-1 controls termination of STING signalling.
Liu Y, Xu P, Rivara S, Liu C, Ricci J, Ren X, Hurley JH, Ablasser A. Liu Y, et al. Nature. 2022 Oct;610(7933):761-767. doi: 10.1038/s41586-022-05354-0. Epub 2022 Oct 19. Nature. 2022. PMID: 36261523 Free PMC article. - Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity.
Fölsch H. Fölsch H. Cell Logist. 2015 Jul 30;5(2):e1074331. doi: 10.1080/21592799.2015.1074331. eCollection 2015 Apr-Jun. Cell Logist. 2015. PMID: 27057418 Free PMC article. Review.
References
- Bonifacino J. S., Traub L. M. (2003) Annu. Rev. Biochem. 72, 395–447 - PubMed
- Robinson M. S. (2004) Trends Cell Biol. 14, 167–174 - PubMed
- Traub L. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 583–596 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials