IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells - PubMed (original) (raw)
. 2010 Dec 20;207(13):2895-906.
doi: 10.1084/jem.20100064. Epub 2010 Nov 22.
Nandhini Ramamoorthi, Noelyn M Kljavin, Cindy S Ma, Jennifer H Cox, Hart S Dengler, Dimitry M Danilenko, Patrick Caplazi, Melanie Wong, David A Fulcher, Matthew C Cook, Cecile King, Stuart G Tangye, Frederic J de Sauvage, Nico Ghilardi
Affiliations
- PMID: 21098093
- PMCID: PMC3005229
- DOI: 10.1084/jem.20100064
IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells
Marcel Batten et al. J Exp Med. 2010.
Abstract
Maturation and selection of high-affinity B cell clones in the germinal center (GC) relies on support from T follicular helper (T(FH)) cells. T(FH) cells are characterized by their localization to the B cell follicle and their high expression of the costimulatory molecules ICOS and PD1 and the cytokine IL-21, which promotes immunoglobulin (Ig) class switching and production by B cells. We show that the heterodimeric cytokine IL-27 is critical for the function of T(FH) cells and for normal and pathogenic GC responses. IL-27 signaling to T cells results in the production of IL-21, a known autocrine factor for the maintenance of T(FH) cells, in a STAT3-dependent manner. IL-27 also enhances the survival of activated CD4(+) T cells and the expression of T(FH) cell phenotypic markers. In vivo, expression of the IL-27Rα chain is required to support IL-21 production and T(FH) cell survival in a T cell-intrinsic manner. The production of high-affinity antibodies is reduced, and pristane-elicited autoantibodies and glomerulonephritis are significantly diminished, in Il27ra(-/-) mice. Together, our data show a nonredundant role for IL-27 in the development of T cell-dependent antibody responses.
Figures
Figure 1.
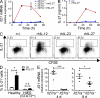
IL-27 induces IL-21 expression. (A) FACS-purified CD4+CD25− T cells isolated from either Il27ra+/+ or Il27ra−/− mice were stimulated with plate-bound anti-CD3 and soluble anti-CD28 under TH0 polarizing conditions and in the presence or absence of 20 ng/ml rmIL-27 for the times indicated. Il21 mRNA was determined by real-time RT-PCR and is presented relative to rpl19 mRNA. Data represent the mean of triplicate experimental samples and error bars indicate standard deviation. (B) CD4+ T cells isolated from C57BL6 spleens were stimulated as in A (n.t., no treatment) and IL-21 protein was measured in the culture supernatant by ELISA. Error bars indicate standard deviation. (C) FACS-purified CD4+CD45RA+CXCR5− cells from human tonsil were labeled with CFSE and stimulated with anti-CD3 + anti-CD28 + anti-CD2 beads (2:1 ratio of beads to cells) in the presence of no additional cytokine, 20 ng/ml rhIL-12 or IL-23, or 50 ng/ml rhIL-27 for 5 d. IL-21 expression was assessed by intracellular staining and flow cytometry and is plotted against CFSE division. The percentage of IL-21–expressing cells is indicated. Similar data were obtained from three individuals. (D) Naive CD4+ T cells were FACS purified from three healthy controls and three AD-HIES patients and stimulated as in C. The mean percentage of IL-21+ cells ± SEM is given. (E) Groups of Il27ra+/+ (filled circles) and Il27ra−/− (open squares) mice were immunized with OVA (30 µg/mouse) in CFA. 4 and 8 d after immunization, CD4+ T cells were isolated from the spleens and IL-21 mRNA was determined by real time RT-PCR (relative to rpl19). Data from individual animals are shown. Bars indicate the mean of five animals ± SEM. *, P < 0.05; ***, P < 0.001 (unpaired Student’s t test). One of three (A and B) or two (E) independent experiments is shown. C shows a single donor representative of six. D shows data combined from three independent donors.
Figure 2.
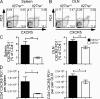
Il27ra−/− mice contain fewer TFH cells than Il27ra+/+ mice. Groups of Il27ra+/+ and Il27ra−/− mice were immunized twice with TNP-OVA in adjuvant and, 7 d after the second immunization, tissue was collected for analysis. (A and B) Representative flow cytometric analysis of CXCR5 and PD1 expression in the CD4+B220− gate in the spleen (A) and DLN (B). (C) The mean percentage ± SEM of CXCR5+PD1+ cells in each spleen or pair of DLN. (D) The numbers of CXCR5+PD1+ cells per organ were calculated for spleen and DLN by multiplying the percentage obtained by flow cytometry by the total cell count per organ. The mean of at least six animals per group is given and error bars indicate SEM. *, P < 0.05 (unpaired Student’s t test). These data are representative of four individual experiments.
Figure 3.
GC dysfunction in Il27ra−/− mice. Groups of Il27ra+/+ and Il27ra−/− mice were immunized twice with TNP-OVA in adjuvant and, 7 d after the second immunization, tissues and sera were collected for analysis. (A) Representative flow cytometric analysis for Fas and GL7 expression in the splenic B220+CD4− cell gate. (B) The number of GL7+Fas+B220+CD4− cells in the spleen of each mouse was calculated by multiplying the percentage obtained by flow cytometry by the total cell count per organ. The mean of at least six animals per group is given and error bars indicate SEM. (C) Representative spleen sections stained with PNA (brown) to detect GC. Bars, 250 µm (D) Slide scanning and image analysis software were used to quantify the PNA+ area in each of eight spleens per genotype. The mean percentage of PNA+ area is given and error bars indicate SEM. (E–G) ELISA using plates coated with 5 µg/ml BSA-TNP28 (E) or BSA-TNP2 (F and G) for analysis of total anti-TNP and high-affinity anti-TNP antibodies, respectively, in the serum of mice immunized as in A. Anti-TNP antibodies were detected with either anti–mouse Ig (E and F) or antibodies against specific mouse Ig isotypes (G). (H) Groups of Il27ra+/+ and Il27ra−/− mice were immunized with 100 µg TNP-Ficoll i.p. and sera were collected 5 d later. Anti–TNP-IgM levels were assessed by ELISA as in E and detected using anti–mouse IgM antibodies. Relative anti-TNP antibody concentration is given for each mouse, and bars indicate the group mean where n = 6–8. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test). The data are indicative of three individual experiments.
Figure 4.
The GC defect in Il27ra−/− mice is T cell intrinsic. (A and B) WT (CD45.1+):Il27ra−/− (CD45.2+) BM chimeric mice were immunized twice with TNP-OVA, as described, and tissue collected and assessed 7 d after the second injection. The ratio of CD45.1/CD45.2 cells is given for total CD4+ cells and CD4+CXCR5+PD1+ cells in the spleen (A) or DLN (B) for each of 10 chimeric animals. The gray dashed line indicates equivalency of WT and Il27ra−/− cells (ratio of 1). (C and D) BM chimeric mice were reconstituted with BM from WT mice only, Il27ra−/− mice only, WT and µMT mice (1:4 ratio), or Il27ra−/− and µMT mice (1:4 ratio) and immunized twice as described. Tissue was collected 7 d after the second injection and the percentage of CXCR5+PD1+ cells in the CD4+B220_−_ gate in the spleen (C) and DLN (D) was determined by flow cytometry. (E) Serum high-affinity antibody was measured using ELISA plates coated with TNP2-BSA and detected with anti–mouse IgG antibody. Data are given as a relative concentration using pooled serum as a control. Each set of data are indicative of two individual experiments. Bars indicate the mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test).
Figure 5.
IL-27 promotes survival of TFH cells. (A) OTII Tg CD4+ T cells were cultured with irradiated splenic APC plus 0.3 µM OVA323-339 peptide under TH0 conditions and in the presence of either no additional cytokine or various concentrations of rmIL-21 or rmIL-27 for 5 d. After 5 d, CD4+ T cells were restimulated with anti-CD3 for 24 h and CXCR5, PD1, and ICOS expression were assessed by flow cytometry. The positive gate was determined based on histogram plots for each antibody and the mean percentage positive ± SD for triplicate experimental samples is shown. (B) DO11.10 tg.rag2−/− splenocytes were activated with 0.03 µM OVA323-339 in the presence or absence (n.t.) of rmIL27 for 72 h, and viability was assessed by flow cytometry using annexin V and 7AAD staining. The CD4+B220− gate is shown. (C and D) Viability of cells in groups of Il27ra+/+ and Il27ra−/− mice was assessed by flow cytometry 4 d after immunization with TNP-OVA in CFA. (C) Representative plots for annexin V staining in the CD4+B220−7AAD− gate (Il27ra+/+, black fill; Il27ra−/−, gray fill) or CXCR5+PD1+CD4+B220−7AAD− cell gate (Il27ra+/+, dotted line; Il27ra−/−, solid line) in the spleen or DLN. (D) The mean percentage of annexin V+ cells in the CD4+CXCR5+PD1+7AAD− gate. Error bars indicate SEM with n = 5 mice per group. Each set of data are indicative of two individual experiments. **, P < 0.01 (unpaired Student’s t test).
Figure 6.
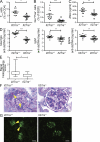
The deletion of Il27ra ameliorates pathology in the pristane-induced lupus model. A single 0.5-ml i.p. injection of pristane was administered to groups of Il27ra+/+ and Il27ra−/− mice. 42 wk after induction, mice were euthanized and examined for immune activation parameters and lesions consistent with SLE. (A and B) Flow cytometric analysis of splenocytes. The percentages of CXCR5+PD1+ (TFH-like) cells in the CD4+B220− gate (A) and the percentages of GL7+Fas+ (GC B cells) in the B220+CD4− gate (B) were analyzed. (C) Serum anti-nuclear antibody (ANA). (D) Serum total IgG (left), IgG1 (middle), and IgG2a (right) anti-dsDNA antibody titers. In all graphs bars indicate mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired Student’s t test). (E and F) Kidney sections were stained with PAS and the glomerulopathy scored by blinded observers. (E) Mean renal lesion scores are depicted as a box plot with max to min whiskers. *, P < 0.05 (unpaired Student’s t test). (F) Representative sections. Arrowheads, lumpy accumulations of PAS-positive material. Bars, 20 µm. (G) Immunofluorescence for IgG immune complex deposition in the kidney. Lumpy IgG deposits (arrowheads) correspond to PAS positive mesangial deposits. Bars, 50 µm. Data represent one experiment with 16 animals per group.
Similar articles
- Transcription factor IRF4 determines germinal center formation through follicular T-helper cell differentiation.
Bollig N, Brüstle A, Kellner K, Ackermann W, Abass E, Raifer H, Camara B, Brendel C, Giel G, Bothur E, Huber M, Paul C, Elli A, Kroczek RA, Nurieva R, Dong C, Jacob R, Mak TW, Lohoff M. Bollig N, et al. Proc Natl Acad Sci U S A. 2012 May 29;109(22):8664-9. doi: 10.1073/pnas.1205834109. Epub 2012 May 2. Proc Natl Acad Sci U S A. 2012. PMID: 22552227 Free PMC article. - Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans.
Flynn R, Du J, Veenstra RG, Reichenbach DK, Panoskaltsis-Mortari A, Taylor PA, Freeman GJ, Serody JS, Murphy WJ, Munn DH, Sarantopoulos S, Luznik L, Maillard I, Koreth J, Cutler C, Soiffer RJ, Antin JH, Ritz J, Dubovsky JA, Byrd JC, MacDonald KP, Hill GR, Blazar BR. Flynn R, et al. Blood. 2014 Jun 19;123(25):3988-98. doi: 10.1182/blood-2014-03-562231. Epub 2014 May 12. Blood. 2014. PMID: 24820310 Free PMC article. - Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150).
Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Yusuf I, et al. J Immunol. 2010 Jul 1;185(1):190-202. doi: 10.4049/jimmunol.0903505. Epub 2010 Jun 4. J Immunol. 2010. PMID: 20525889 Free PMC article. - Follicular helper T cells poise immune responses to the development of autoimmune pathology.
Gómez-Martín D, Díaz-Zamudio M, Romo-Tena J, Ibarra-Sánchez MJ, Alcocer-Varela J. Gómez-Martín D, et al. Autoimmun Rev. 2011 Apr;10(6):325-30. doi: 10.1016/j.autrev.2010.11.007. Epub 2010 Dec 15. Autoimmun Rev. 2011. PMID: 21167320 Review. - The darker side of follicular helper T cells: from autoimmunity to immunodeficiency.
Shekhar S, Yang X. Shekhar S, et al. Cell Mol Immunol. 2012 Sep;9(5):380-5. doi: 10.1038/cmi.2012.26. Epub 2012 Aug 13. Cell Mol Immunol. 2012. PMID: 22885524 Free PMC article. Review.
Cited by
- Interleukin-27 in T cell immunity.
Iwasaki Y, Fujio K, Okamura T, Yamamoto K. Iwasaki Y, et al. Int J Mol Sci. 2015 Jan 27;16(2):2851-63. doi: 10.3390/ijms16022851. Int J Mol Sci. 2015. PMID: 25633106 Free PMC article. Review. - C-type lectin receptors in the control of T helper cell differentiation.
Geijtenbeek TB, Gringhuis SI. Geijtenbeek TB, et al. Nat Rev Immunol. 2016 Jul;16(7):433-48. doi: 10.1038/nri.2016.55. Epub 2016 Jun 13. Nat Rev Immunol. 2016. PMID: 27291962 Review. - RACK1 enhances STAT3 stability and promotes T follicular helper cell development and function during blood-stage Plasmodium infection in mice.
Cheng Q, Yang X, Zou T, Sun L, Zhang X, Deng L, Wu M, Gai W, Jiang H, Guo T, Lu Y, Dong J, Niu C, Pan W, Zhang J. Cheng Q, et al. PLoS Pathog. 2024 Jul 18;20(7):e1012352. doi: 10.1371/journal.ppat.1012352. eCollection 2024 Jul. PLoS Pathog. 2024. PMID: 39024388 Free PMC article. - The Yin and Yang aspects of IL-27 in induction of cancer-specific T-cell responses and immunotherapy.
Li MS, Liu Z, Liu JQ, Zhu X, Liu Z, Bai XF. Li MS, et al. Immunotherapy. 2015;7(2):191-200. doi: 10.2217/imt.14.95. Immunotherapy. 2015. PMID: 25713993 Free PMC article. Review. - IL-27 is elevated in patients with COPD and patients with pulmonary TB and induces human bronchial epithelial cells to produce CXCL10.
Cao J, Zhang L, Li D, Xu F, Huang S, Xiang Y, Yin Y, Ren G. Cao J, et al. Chest. 2012 Jan;141(1):121-130. doi: 10.1378/chest.10-3297. Epub 2011 Jul 21. Chest. 2012. PMID: 21778255 Free PMC article.
References
- Batten M., Kljavin N.M., Li J., Walter M.J., de Sauvage F.J., Ghilardi N. 2008. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J. Immunol. 180:2752–2756 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous