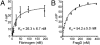Alzheimer's disease peptide beta-amyloid interacts with fibrinogen and induces its oligomerization - PubMed (original) (raw)
Alzheimer's disease peptide beta-amyloid interacts with fibrinogen and induces its oligomerization
Hyung Jin Ahn et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Increasing evidence supports a vascular contribution to Alzheimer's disease (AD), but a direct connection between AD and the circulatory system has not been established. Previous work has shown that blood clots formed in the presence of the β-amyloid peptide (Aβ), which has been implicated in AD, have an abnormal structure and are resistant to degradation in vitro and in vivo. In the present study, we show that Aβ specifically interacts with fibrinogen with a K(d) of 26.3 ± 6.7 nM, that the binding site is located near the C terminus of the fibrinogen β-chain, and that the binding causes fibrinogen to oligomerize. These results suggest that the interaction between Aβ and fibrinogen modifies fibrinogen's structure, which may then lead to abnormal fibrin clot formation. Overall, our study indicates that the interaction between Aβ and fibrinogen may be an important contributor to the vascular abnormalities found in AD.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
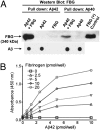
Aβ peptide and fibrinogen interact in vitro. (A) Biotinylated Aβ42 or Aβ40 was incubated with fibrinogen (FBG) and pull-down assays were carried out using streptavidin-Sepharose. All samples were analyzed by Western blot and are representative of multiple experiments. A Western blot was performed under nonreducing conditions by using an anti-fibrinogen antibody, showing that Aβ42 and Aβ40 bind to fibrinogen. Dot blots against Aβ below each pull-down show that comparable amounts of biotinylated Aβ were pulled down. (B) The binding curve of biotin-labeled Aβ42 and fibrinogen was determined by ELISA. When no Aβ42 was added, the level of bound fibrinogen was negligible, regardless of fibrinogen concentration. Therefore, there is no nonspecific binding between fibrinogen and streptavidin in the well.
Fig. 2.
Aβ42 binds to FragD of fibrinogen. Biotinylated Aβ42 was incubated with (A) FragD (FD) or (B) FragE (FE), and pull-down assays were carried out using streptavidin-Sepharose. A Western blot was performed using an anti-fibrinogen antibody. Dot blots against Aβ showed that comparable amounts of Aβ were pulled down. Binding curve of biotin-labeled Aβ42 and FragD or FragE was determined by ELISA. We observed an Aβ42-dependent increase in binding of FragD (C), but no binding between Aβ42 and FragE (D). These results represent multiple experiments.
Fig. 3.
The interaction of Aβ42 with fibrinogen or FragD is concentration-dependent. The binding kinetics between Aβ42 and fibrinogen (A) or FragD (B) were monitored by using FP. Increasing concentrations of fibrinogen or FragD were incubated with TAMRA-labeled Aβ42. After the reaction reached equilibrium, polarization was measured, and _K_d was calculated by fitting the data to a single-site binding equation (n = 3). The _K_d of Aβ42 binding to fibrinogen (two sites) was less than that to FragD (one site), and the data fit well to a one-site binding model. When the data are fitted to a two-site binding model, the dissociation constants for the two sites are virtually identical. It is likely that the distance between the two binding sites on fibrinogen may allow them to bind Aβ independently of each other.
Fig. 4.
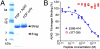
Aβ42 specifically interacts with fibrinogen near the C terminus of the fibrinogen β-chain. (A) FDPs were incubated with biotinylated Aβ42 followed by streptavidin-Sepharose beads. Fragments interacting with Aβ42 were eluted from the beads and analyzed using SDS/PAGE. Reaction mixtures lacking biotinylated Aβ42 served as a control for nonspecific binding (“FDP only” lane). The major band below 16 kDa is streptavidin (Strep). A fragment interacting with Aβ42 (Frag) was identified around the 7 kDa protein size marker. (B) The inhibition curve of the fibrinogen-derived 49-mer peptide (β366–β414) for the Aβ42–fibrinogen interaction was measured using AlphaLISA. The 49-mer peptide had an IC50 of 0.99 ± 00.1μM, whereas fibrinogen peptide γ377–γ395 did not show any inhibition of the Aβ42–fibrinogen interaction (n = 3).
Fig. 5.
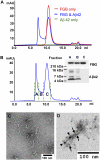
Aβ42 binding induces oligomerization of fibrinogen. Fibrinogen (FBG; 1 μM) plus Aβ42 (blue; 5 μM), FBG alone (red; 1 μM), or Aβ42 alone (green; 5 μM) were incubated for 2 h at 37 °C. (A) Samples were run on a Superose 6 gel filtration column on FPLC. (B) Eluates from the Aβ42–fibrinogen mixture were analyzed by Western blot using an antibody against FBG or Aβ42. (C and D) TEM images were obtained from fibrinogen oligomers purified using size-exclusion chromatography. Immunogold labeling shows that oligomer is composed of both Aβ and fibrinogen (D). Aβ42-fibrinogen oligomers were labeled with anti-fibrinogen antibody (6 nm gold; arrow) and anti-Aβ antibody (12 nm gold; arrowhead). This result represents multiple experiments.
Fig. 6.
Proposed model for the role of Aβ42 and fibrinogen binding in AD pathology. The Aβ peptide interacts with fibrinogen and modifies the formation of fibrin clots. These altered clots have an abnormal structure and are resistant to degradation by fibrinolytic enzymes. In patients with AD, persistent fibrin(ogen) could lead to vascular deficiencies, decreased blood flow, cognitive dysfunction, and neuroinflammation. (Scale bar: 50 nm in TEM image of fibrinogen oligomer.)
Similar articles
- Hyperhomocysteinemia exacerbates Alzheimer's disease pathology by way of the β-amyloid fibrinogen interaction.
Chung YC, Kruyer A, Yao Y, Feierman E, Richards A, Strickland S, Norris EH. Chung YC, et al. J Thromb Haemost. 2016 Jul;14(7):1442-52. doi: 10.1111/jth.13340. Epub 2016 Jun 13. J Thromb Haemost. 2016. PMID: 27090576 Free PMC article. - Epitope Mapping Immunoassay Analysis of the Interaction between β-Amyloid and Fibrinogen.
Van Giau V, An SSA. Van Giau V, et al. Int J Mol Sci. 2019 Jan 24;20(3):496. doi: 10.3390/ijms20030496. Int J Mol Sci. 2019. PMID: 30678343 Free PMC article. - Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer's disease.
Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, Bhuvanendran S, Fenz KM, Strickland S. Cortes-Canteli M, et al. Neuron. 2010 Jun 10;66(5):695-709. doi: 10.1016/j.neuron.2010.05.014. Neuron. 2010. PMID: 20547128 Free PMC article. - Interactions of β-amyloid peptide with fibrinogen and coagulation factor XII may contribute to Alzheimer's disease.
Ahn HJ, Chen ZL, Zamolodchikov D, Norris EH, Strickland S. Ahn HJ, et al. Curr Opin Hematol. 2017 Sep;24(5):427-431. doi: 10.1097/MOH.0000000000000368. Curr Opin Hematol. 2017. PMID: 28661939 Free PMC article. Review. - Fibrinogen and altered hemostasis in Alzheimer's disease.
Cortes-Canteli M, Zamolodchikov D, Ahn HJ, Strickland S, Norris EH. Cortes-Canteli M, et al. J Alzheimers Dis. 2012;32(3):599-608. doi: 10.3233/JAD-2012-120820. J Alzheimers Dis. 2012. PMID: 22869464 Free PMC article. Review.
Cited by
- Cerebrovascular disorders caused by hyperfibrinogenaemia.
Muradashvili N, Tyagi R, Tyagi N, Tyagi SC, Lominadze D. Muradashvili N, et al. J Physiol. 2016 Oct 15;594(20):5941-5957. doi: 10.1113/JP272558. Epub 2016 Jun 16. J Physiol. 2016. PMID: 27121987 Free PMC article. - Fibrinogen-cellular prion protein complex formation on astrocytes.
Charkviani M, Muradashvili N, Sulimai N, Lominadze D. Charkviani M, et al. J Neurophysiol. 2020 Aug 1;124(2):536-543. doi: 10.1152/jn.00224.2020. Epub 2020 Jul 22. J Neurophysiol. 2020. PMID: 32697670 Free PMC article. - Hyperfibrinogenemia-mediated astrocyte activation.
Clark VD, Layson A, Charkviani M, Muradashvili N, Lominadze D. Clark VD, et al. Brain Res. 2018 Nov 15;1699:158-165. doi: 10.1016/j.brainres.2018.08.023. Epub 2018 Aug 25. Brain Res. 2018. PMID: 30153459 Free PMC article. - A Leaky Blood-Brain Barrier to Fibrinogen Contributes to Oxidative Damage in Alzheimer's Disease.
McLarnon JG. McLarnon JG. Antioxidants (Basel). 2021 Dec 31;11(1):102. doi: 10.3390/antiox11010102. Antioxidants (Basel). 2021. PMID: 35052606 Free PMC article. Review. - Direct Oral Anticoagulants (DOACs) for Therapeutic Targeting of Thrombin, a Key Mediator of Cerebrovascular and Neuronal Dysfunction in Alzheimer's Disease.
Grossmann K. Grossmann K. Biomedicines. 2022 Aug 4;10(8):1890. doi: 10.3390/biomedicines10081890. Biomedicines. 2022. PMID: 36009437 Free PMC article. Review.
References
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
- Cleary JP, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. - PubMed
- Glenner GG, Wong CW. Alzheimer's disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
- Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 1966;209:109–110. - PubMed
- Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer's disease. Acta Neuropathol. 2008;115:599–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases