A proton current drives action potentials in genetically identified sour taste cells - PubMed (original) (raw)
A proton current drives action potentials in genetically identified sour taste cells
Rui B Chang et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Five tastes have been identified, each of which is transduced by a separate set of taste cells. Of these sour, which is associated with acid stimuli, is the least understood. Genetic ablation experiments have established that sour is detected by a subset of taste cells that express the TRP channel PKD2L1 and its partner PKD1L3, however the mechanisms by which this subset of cells detects acids remain unclear. Previous efforts to understand sour taste transduction have been hindered because sour responsive cells represent only a small fraction of cells in a taste bud, and numerous ion channels with no role in sour sensing are sensitive to acidic pH. To identify acid-sensitive conductances unique to sour cells, we created genetically modified mice in which sour cells were marked by expression of YFP under the control of the PKD2L1 promoter. To measure responses to sour stimuli we developed a method in which suction electrode recording is combined with UV photolysis of NPE-caged proton. Using these methods, we report that responses to sour stimuli are not mediated by Na(+) permeable channels as previously thought, but instead are mediated by a proton conductance specific to PKD2L1-expressing taste cells. This conductance is sufficient to drive action potential firing in response to acid stimuli, is enriched in the apical membrane of PKD2L1-expressing taste cells and is not affected by targeted deletion of the PKD1L3 gene. We conclude that, during sour transduction, protons enter through an apical proton conductance to directly depolarize the taste cell membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
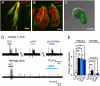
Fig. 1.
YFP expression driven by the PKD2L1 promoter faithfully marks sour-responsive taste receptor cells. (A and B) In circumvallate papillae, YFP (green) is expressed by taste cells that are immunoreactive for PKD2L1 (red, A), but not by cells immunoreactive for TRPM5 (red, B). (C) Acutely dissociated taste cells from PKD2L1-YFP mice showed intense YFP fluorescence. (D) Action potentials recorded from a PKD2L1-YFP cell (Upper) and a TRPM5-GFP cell (Lower). Solutions at pH 6 were buffered with either 10 mM Mes or 10 mM acetic acid (HOAc). (E) Average data from experiments as in D. Control (con) was measured in the 10-s interval before KCl (25-mM) stimulation. Data represent mean ± SEM; **P < 0.01; ***P < 0.001. (Scale bars: A and B, 10 μm; C, 5 μm.)
Fig. 2.
Extracellular acidification specifically evokes an inward current in PKD2L1-YFP cells. (A) Whole-cell current at −80 mV from a PKD2L1-YFP taste cell in response to HCl pH 5 (10 mM Mes) or HOAc, pH 5 (2 mM) in the presence of extracellular Na+. (B) Replacement of extracellular Na+ with NMDG+ did not affect the inward current at −80 mV in response to pH 5 (HCl). I-V relationship measured at the time points indicated are shown at Right. (C) Average magnitude of the acid-induced current at −80 mV from experiments as in A and B. Average reversal potential (Er) of the total current under each condition is shown at Right (n = 3–15). Note that the response is indistinguishable between HCl (Mes) and HOAc, and manipulations of the major ions (Na+, Ca2+, and Cl−) had no effect (P > 0.17 for all comparisons of current magnitude with control and P > 0.14 for all comparisons of Er with control). Data represent the mean ± SEM. **P < 0.01.
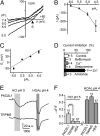
Fig. 3.
The acid-evoked inward current in PKD2L1-YFP cells is conducted by protons. (A) Current–voltage relationship from a PKD2L1-YFP cell in response to pH 6, 5.5, and 5 (HCl) solutions under Na+-free conditions. (B and C) Average magnitude (at −80 mV) and reversal potential of the currents from experiments as in A (n = 3–15). Dashed line shows a linear fit of the data with a slope of 51.0 ± 3.8 mV/pH. (D) Effect of indicated chemicals on current evoked by pH 5 (HCl) at −80 mV. Concentrations were as follows:1 mM CdCl2; 100 μM desipramine; 200 nM Bafilomycin A1; 1 mM ZnCl2; and 30 μM amiloride. Block was measured as magnitude of current after incubation with test chemical for 10 s, as compared with magnitude of current immediately before application. No chemicals were given in the control group, and the ∼10% block represents normal rundown of current in 10 s. (E) Change in intracellular pH, as indicated by fluorescence intensity of carboxy-DFFDA, in response to pH 5 (HCl) and pH 4 (20 mM HOAc) solutions in a PKD2L1-YFP (Upper) and a TRPM5-GFP (Lower) taste cell. (F) Average data from experiments as in E. Data represent mean ± SEM. ***P < 0.001.
Fig. 4.
Uncaging protons at the apical surface of PKD2L1-YFP cells evokes a receptor current and action potentials. (A) Apical membrane of a PKD2L1-YFP taste receptor cell was drawn into a suction pipette containing 2 mM NPE-caged proton. (Scale bar, 10 μm.) In this mode, the cell membrane is intact and acid stimuli can be selectively delivered to the apical surface. (B) Control experiments showing that UV uncaging of NPE-caged proton in patch pipette in cell-attached mode evoked endogenous ASIC currents in untransfected HEK 293 cells (Upper) and TRPV1 currents in HEK 293 cells transfected with TRPV1 (Lower). (C) Membrane current and action potentials recorded from taste cells upon UV uncaging of NPE-caged proton in pipette as in A. PKD2L1-YFP, but not TRPM5-GFP, cells responded to apical delivery of protons with a large inward current and the generation of a train of action potentials. Other traces show effects of changing the composition of the pipette solution or of recording from the basolateral surface. Average data are shown at Right. Blue bars represent magnitude of currents evoked by the UV flash; red bars represent the rate of evoked action potentials. Data represent mean ± SEM. **P < 0.01, ***P < 0.001, as compared with responses from apical surface with Na+ in pipette except for Zn2+, for which the control was with NMDG+ in the pipette. Responses with NMDG+ in the pipette were not significantly different (n.s.) from responses with Na+ in the pipette (P = 0.64 and P = 0.13 for current and rate of APs, respectively).
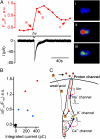
Fig. 5.
Elevation of intracellular Ca2+ in response to apical delivery of protons in PKD2L1-YFP cells. (A) Simultaneous measurement of the change in intracellular Ca2+ (Upper Left) and the magnitude of current (Lower Left) in response to apical uncaging of NPE-caged proton in a PKD2L1-YFP cell. Fluorescent images taken at different time points are shown at Right. (B) Scatter plot of the change in intracellular Ca2+ as a function of the integrated current in response to UV uncaging from experiments as in A. Red, cells that fired action potentials; blue, cells that did not fire action potentials; black, control data measured 10 s before the UV flash. (C) Proposed model for sour taste transduction in PKD2L1-expressing cells. Proton entry through a proton-selective conductance specifically expressed on apical surface of PKD2L1-expressing cells leads to depolarization and generation of action potentials that propagate to the cell body and activate voltage-gated Ca2+ channels. In addition to accessing this pathway, weak acids may produce intracellular acidification and inhibit resting K+ conductances.
Comment in
- The sour taste of a proton current.
Frings S. Frings S. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):21955-6. doi: 10.1073/pnas.1016810108. Epub 2010 Dec 13. Proc Natl Acad Sci U S A. 2010. PMID: 21149726 Free PMC article. No abstract available.
Similar articles
- The sour taste of a proton current.
Frings S. Frings S. Proc Natl Acad Sci U S A. 2010 Dec 21;107(51):21955-6. doi: 10.1073/pnas.1016810108. Epub 2010 Dec 13. Proc Natl Acad Sci U S A. 2010. PMID: 21149726 Free PMC article. No abstract available. - Activation of polycystic kidney disease-2-like 1 (PKD2L1)-PKD1L3 complex by acid in mouse taste cells.
Kawaguchi H, Yamanaka A, Uchida K, Shibasaki K, Sokabe T, Maruyama Y, Yanagawa Y, Murakami S, Tominaga M. Kawaguchi H, et al. J Biol Chem. 2010 Jun 4;285(23):17277-81. doi: 10.1074/jbc.C110.132944. Epub 2010 Apr 20. J Biol Chem. 2010. PMID: 20406802 Free PMC article. - A proton current associated with sour taste: distribution and functional properties.
Bushman JD, Ye W, Liman ER. Bushman JD, et al. FASEB J. 2015 Jul;29(7):3014-26. doi: 10.1096/fj.14-265694. Epub 2015 Apr 9. FASEB J. 2015. PMID: 25857556 Free PMC article. - Role of apical ion channels in sour taste transduction.
Kinnamon SC. Kinnamon SC. Ciba Found Symp. 1993;179:201-10; discussion 210-7. doi: 10.1002/9780470514511.ch13. Ciba Found Symp. 1993. PMID: 7513271 Review. - Transient receptor potential (TRP) channels and taste sensation.
Ishimaru Y, Matsunami H. Ishimaru Y, et al. J Dent Res. 2009 Mar;88(3):212-8. doi: 10.1177/0022034508330212. J Dent Res. 2009. PMID: 19329452 Free PMC article. Review.
Cited by
- Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster.
Charlu S, Wisotsky Z, Medina A, Dahanukar A. Charlu S, et al. Nat Commun. 2013;4:2042. doi: 10.1038/ncomms3042. Nat Commun. 2013. PMID: 23783889 Free PMC article. - Synaptic communication and signal processing among sensory cells in taste buds.
Chaudhari N. Chaudhari N. J Physiol. 2014 Aug 15;592(16):3387-92. doi: 10.1113/jphysiol.2013.269837. Epub 2014 Mar 24. J Physiol. 2014. PMID: 24665098 Free PMC article. - Sour taste finds closure in a potassium channel.
Challis RC, Ma M. Challis RC, et al. Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):246-7. doi: 10.1073/pnas.1523319113. Epub 2015 Dec 30. Proc Natl Acad Sci U S A. 2016. PMID: 26719421 Free PMC article. No abstract available. - Acid-inducible proton influx currents in the plasma membrane of murine osteoclast-like cells.
Kuno M, Li G, Moriura Y, Hino Y, Kawawaki J, Sakai H. Kuno M, et al. Pflugers Arch. 2016 May;468(5):837-47. doi: 10.1007/s00424-016-1796-7. Epub 2016 Feb 3. Pflugers Arch. 2016. PMID: 26843093 - Proton production, regulation and pathophysiological roles in the mammalian brain.
Zeng WZ, Xu TL. Zeng WZ, et al. Neurosci Bull. 2012 Feb;28(1):1-13. doi: 10.1007/s12264-012-1068-2. Neurosci Bull. 2012. PMID: 22233885 Free PMC article. Review.
References
- Lyall V, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281:C1005–C1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DC004564/DC/NIDCD NIH HHS/United States
- R01 DC004564-12/DC/NIDCD NIH HHS/United States
- R01 DC004564-13/DC/NIDCD NIH HHS/United States
- DC004564/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases