Modulation of E-cadherin function and dysfunction by N-glycosylation - PubMed (original) (raw)
Review
Modulation of E-cadherin function and dysfunction by N-glycosylation
Salomé S Pinho et al. Cell Mol Life Sci. 2011 Mar.
Abstract
Several mechanisms have been proposed to explain the E-cadherin dysfunction in cancer, including genetic and epigenetic alterations. Nevertheless, a significant number of human carcinomas have been seen that show E-cadherin dysfunction that cannot be explained at the genetic/epigenetic level. A substantial body of evidence has appeared recently that supports the view that other mechanisms operating at the post-translational level may also affect E-cadherin function. The present review addresses molecular aspects related to E-cadherin N-glycosylation and evidence is presented showing that the modification of N-linked glycans on E-cadherin can affect the adhesive function of this adhesion molecule. The role of glycosyltransferases involved in the remodeling of N-glycans on E-cadherin, including N-acetylglucosaminyltransferase III (GnT-III), N-acetylglucosaminyltransferase V (GnT-V), and the α1,6 fucosyltransferase (FUT8) enzyme, is also discussed. Finally, this review discusses an alternative functional regulatory mechanism for E-cadherin operating at the post-translational level, N-glycosylation, that may underlie the E-cadherin dysfunction in some carcinomas.
Figures
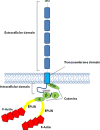
Fig. 1
Schematic representation of the E-cadherin–catenin complex. The E-cadherin–catenin complex is proposed to interact with F-actin via α-catenin association with actin-binding proteins such as EPLIN [5]. β-Catenin and γ-catenin bind to E-cadherin in a mutually exclusive manner
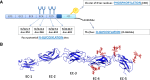
Fig. 2
E-cadherin post-translational modifications. a The extracellular domain (EC) of human E-cadherin contains four potential _N_-glycosylation sites, which are located in EC4 and EC5. The phosphorylation of E-cadherin by casein kinase II (CKII) can occur in a short stretch of 30 aa in the cytoplasmic domain (CD), which contains a cluster of 8 Ser residues. Cytoplasmic _O_-glycosylation (_O_-GlcNAc addition in Thr/Ser residues) has been reported to regulate E-cadherin. These mechanims can modulate E-cadherin mediated cell–cell adhesion at a post-translational level. b Three-dimensional structure of the extracellular domain (EC1–EC5) of E-cadherin. The crystal structure of human EC1 was used in this representation and EC2–EC5 were modeled based on the crystal structure of C-cadherin. Four _N_-glycans were modeled with GlyProt (
http://www.glycosciences.de/glyprot/
), as shown in red
Fig. 3
Biosynthesis of _N_-linked glycans. Representation of a _N_-glycan structure with the reactions catalyzed by GnT-III, GnT-V, and FUT8
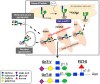
Fig. 4
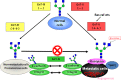
Regulatory mechanism of E-cadherin-mediated cell–cell adhesion and GnT-III/GnT-V. GnT-III activity is associated with an increase in bisecting GlcNAc structures in E-cadherin, leading to a concomitant decrease in β1,6 branched structures, due to competition with GnT-V glycosyltransferase. The addition of bisecting GlcNAc residues to E-cadherin down-regulates the tyrosine phosphorylation of β-catenin and thus enhances cell–cell binding to suppress metastasis (lower figure). Conversely, in metastatic cancer cells (upper figure), the addition of β1,6 branched structures by GnT-V to E-cadherin is associated with increased tyrosine phosphorylation of β-catenin through the EGFR and Src signaling pathways, and therefore reduces E-cadherin-mediated cell–cell adhesion thereby contributing to the promotion of cancer metastasis
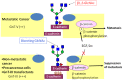
Fig. 5
The role of _N_-glycan structures in the carcinogenic process. In normal cells, the GnT-III and GnT-V enzymes are normally underexpressed. The overexpression of GnT-III is associated with increased synthesis of bisecting GlcNAc structures in some important target glycoproteins involved in cell adhesion such as E-cadherin and integrins, the modification of which by bisecting _N_-glycans is associated with the suppression of metastasis through enhancement of E-cadherin-mediated cell–cell adhesion and a decrease in integrin-mediated cell-extracellular matrix adhesion. Furthermore, GnT-III up-regulation precludes the availability of the substrate for the GnT-V enzyme, which is no longer able to synthesize branched structures. In a metastatic cancer situation, activation of the ras-raf-ets signaling pathway regulates the transcription of the GnT-V gene and the resulting increase in GnT-V leads to increased enzymatic production of β1,6 branched structures that modify glycoproteins involved in the carcinogenic process, including Matriptase; TIMP-1 (Tissue Inhibitor of Metalloproteinase-1, in which β1,6 branching correlates with the invasive and metastatic potential of cancer cells) as well as integrins and E-cadherin, the modification of which contributes to a decrease in cell–cell adhesion, and increase in tumor cell invasion and migration. In addition, other mechanisms also indicate that a secreted type of GnT-V may contribute to tumor angiogenesis
Similar articles
- E-cadherin and adherens-junctions stability in gastric carcinoma: functional implications of glycosyltransferases involving N-glycan branching biosynthesis, N-acetylglucosaminyltransferases III and V.
Pinho SS, Figueiredo J, Cabral J, Carvalho S, Dourado J, Magalhães A, Gärtner F, Mendonfa AM, Isaji T, Gu J, Carneiro F, Seruca R, Taniguchi N, Reis CA. Pinho SS, et al. Biochim Biophys Acta. 2013 Mar;1830(3):2690-700. doi: 10.1016/j.bbagen.2012.10.021. Biochim Biophys Acta. 2013. PMID: 23671930 - The role of N-acetylglucosaminyltransferase III and V in the post-transcriptional modifications of E-cadherin.
Pinho SS, Reis CA, Paredes J, Magalhães AM, Ferreira AC, Figueiredo J, Xiaogang W, Carneiro F, Gärtner F, Seruca R. Pinho SS, et al. Hum Mol Genet. 2009 Jul 15;18(14):2599-608. doi: 10.1093/hmg/ddp194. Epub 2009 Apr 29. Hum Mol Genet. 2009. PMID: 19403558 - Cell-cell interaction-dependent regulation of N-acetylglucosaminyltransferase III and the bisected N-glycans in GE11 epithelial cells. Involvement of E-cadherin-mediated cell adhesion.
Iijima J, Zhao Y, Isaji T, Kameyama A, Nakaya S, Wang X, Ihara H, Cheng X, Nakagawa T, Miyoshi E, Kondo A, Narimatsu H, Taniguchi N, Gu J. Iijima J, et al. J Biol Chem. 2006 May 12;281(19):13038-13046. doi: 10.1074/jbc.M601961200. Epub 2006 Mar 14. J Biol Chem. 2006. PMID: 16537539 - Importance of N-glycosylation on alpha5beta1 integrin for its biological functions.
Gu J, Isaji T, Sato Y, Kariya Y, Fukuda T. Gu J, et al. Biol Pharm Bull. 2009 May;32(5):780-5. doi: 10.1248/bpb.32.780. Biol Pharm Bull. 2009. PMID: 19420742 Review. - Glycans and cancer: role of N-glycans in cancer biomarker, progression and metastasis, and therapeutics.
Taniguchi N, Kizuka Y. Taniguchi N, et al. Adv Cancer Res. 2015;126:11-51. doi: 10.1016/bs.acr.2014.11.001. Epub 2015 Feb 7. Adv Cancer Res. 2015. PMID: 25727145 Review.
Cited by
- Suppression of G6PD induces the expression and bisecting GlcNAc-branched N-glycosylation of E-Cadherin to block epithelial-mesenchymal transition and lymphatic metastasis.
Wang Y, Li Q, Niu L, Xu L, Guo Y, Wang L, Guo C. Wang Y, et al. Br J Cancer. 2020 Oct;123(8):1315-1325. doi: 10.1038/s41416-020-1007-3. Epub 2020 Jul 28. Br J Cancer. 2020. PMID: 32719549 Free PMC article. - Pooled sample-based GWAS: a cost-effective alternative for identifying colorectal and prostate cancer risk variants in the Polish population.
Gaj P, Maryan N, Hennig EE, Ledwon JK, Paziewska A, Majewska A, Karczmarski J, Nesteruk M, Wolski J, Antoniewicz AA, Przytulski K, Rutkowski A, Teumer A, Homuth G, Starzyńska T, Regula J, Ostrowski J. Gaj P, et al. PLoS One. 2012;7(4):e35307. doi: 10.1371/journal.pone.0035307. Epub 2012 Apr 19. PLoS One. 2012. PMID: 22532847 Free PMC article. - Diffuse Gastric Cancer: A Summary of Analogous Contributing Factors for Its Molecular Pathogenicity.
Ansari S, Gantuya B, Tuan VP, Yamaoka Y. Ansari S, et al. Int J Mol Sci. 2018 Aug 16;19(8):2424. doi: 10.3390/ijms19082424. Int J Mol Sci. 2018. PMID: 30115886 Free PMC article. Review. - α-1,2-Mannosidase MAN1C1 Inhibits Proliferation and Invasion of Clear Cell Renal Cell Carcinoma.
Li H, Wang G, Yu Y, Jian W, Zhang D, Wang Y, Wang T, Meng Y, Yuan C, Zhang C. Li H, et al. J Cancer. 2018 Nov 24;9(24):4618-4626. doi: 10.7150/jca.27673. eCollection 2018. J Cancer. 2018. PMID: 30588245 Free PMC article. - Glycosylation and its research progress in endometrial cancer.
Pu C, Biyuan, Xu K, Zhao Y. Pu C, et al. Clin Transl Oncol. 2022 Oct;24(10):1865-1880. doi: 10.1007/s12094-022-02858-z. Epub 2022 Jun 25. Clin Transl Oncol. 2022. PMID: 35752750 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources