A novel non-SET domain multi-subunit methyltransferase required for sequential nucleosomal histone H3 methylation by the mixed lineage leukemia protein-1 (MLL1) core complex - PubMed (original) (raw)
A novel non-SET domain multi-subunit methyltransferase required for sequential nucleosomal histone H3 methylation by the mixed lineage leukemia protein-1 (MLL1) core complex
Anamika Patel et al. J Biol Chem. 2011.
Erratum in
- J Biol Chem. 2011 May 20;286(20):18344
Abstract
Gene expression within the context of eukaryotic chromatin is regulated by enzymes that catalyze histone lysine methylation. Histone lysine methyltransferases that have been identified to date possess the evolutionarily conserved SET or Dot1-like domains. We previously reported the identification of a new multi-subunit histone H3 lysine 4 methyltransferase lacking homology to the SET or Dot1 family of histone lysine methyltransferases. This enzymatic activity requires a complex that includes WRAD (WDR5, RbBP5, Ash2L, and DPY-30), a complex that is part of the MLL1 (mixed lineage leukemia protein-1) core complex but that also exists independently of MLL1 in the cell. Here, we report that the minimal complex required for WRAD enzymatic activity includes WDR5, RbBP5, and Ash2L and that DPY-30, although not required for enzymatic activity, increases the histone substrate specificity of the WRAD complex. We also show that WRAD requires zinc for catalytic activity, displays Michaelis-Menten kinetics, and is inhibited by S-adenosyl-homocysteine. In addition, we demonstrate that WRAD preferentially methylates lysine 4 of histone H3 within the context of the H3/H4 tetramer but does not methylate nucleosomal histone H3 on its own. In contrast, we find that MLL1 and WRAD are required for nucleosomal histone H3 methylation, and we provide evidence suggesting that each plays distinct structural and catalytic roles in the recognition and methylation of a nucleosome substrate. Our results indicate that WRAD is a new H3K4 methyltransferase with functions that include regulating the substrate and product specificities of the MLL1 core complex.
Figures
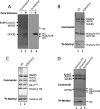
FIGURE 1.
WRAD is a histone H3 lysine 4-specific monomethyltransferase. A, enzymatic assays showing the methyltransferase activity of WRAD after a period of 8 h using [3H]methyl-_S_-adenosylmethionine and purified chicken core histones as substrates. Enzymatic reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (left panel, lanes 1 and 2) or fluorography (right panel, lanes 3 and 4). B, enzymatic activity of WRAD using 4 μg of full-length histone H3 proteins (Active Motif®) that were either unmodified (H3, lane 1) or previously monomethylated at lysine 4 (H3K4me1, lane 2). The reactions were separated by 18% Tris-glycine PAGE and visualized with Coomassie Brilliant Blue (upper panel) and fluorography (3H-Methyl, lower panel). C, enzymatic activity of MLL1 core complex after a period of 8 h using 4 μg of full-length histone H3 proteins that were either unmodified (H3, lane 1) or previously monomethylated at lysine 4 (H3K4me1, lane 2). The reaction products were separated by 4–12% Bis-Tris PAGE and visualized as described in B. D, comparison of the enzymatic activity of WRAD using as substrates recombinant histone H3 (1.5 μg, lane 1), dialyzed histone octamer (6 μg, lane 2), or an equivalent amount of reconstituted nucleosomes (lane 3). The reactions were separated by 18% Tris-glycine SDS-PAGE and visualized as described in B above. Octamer* denotes that the histone octamer dissociates into one H3/H4 tetramer and two H2A/H2B dimers upon dialysis into assay buffer (see text).
FIGURE 2.
WDR5, RbBP5, and Ash2L are required for the H3K4 methylation activity of WRAD. A, comparison of enzymatic activity of individual WRAD components and all possible binary, ternary, and quaternary complexes. W, WDR5; R, RbBP5; A, Ash2L; D, DPY-30. Histone methyltransferase assays were conducted for a period of 8 h using [3H]AdoMet and chicken core histones as the substrate. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panels) and fluorography (lower panels). A no-enzyme control is shown in lane 16. B, WRAD and WRA histone methyltransferase activity as a function of enzyme concentration. Methyltransferase activity assays were conducted with 25 μ
m
[3H]AdoMet and 500 μ
m
histone H3 peptide (residues 1–20) with varying concentrations of WRAD (open triangles) or WRA (open circles). Each point corresponds to the average of duplicate measurements with the error bars indicating the standard error of measurement. Linear regression fitting of the data gave slopes of 0.0016 and 0.001, and _R_2 values of 0.99 and 0.97 for WRAD and WRA complexes, respectively. C, diffusion-free sedimentation coefficient distributions (c(s)) derived from sedimentation velocity analytical ultracentrifugation of WRAD (upper panel) and WRA (lower panel) complexes at concentrations of 2.2 μ
m
(solid line), 1.1 μ
m
(dashed line), and 0.55 μ
m
(dotted line).
FIGURE 3.
Steady-state kinetics and inhibition analysis of WRA(D). A, comparison of WRA (open circle) and WRAD (open triangle) kinetics with AdoMet as the variable substrate (ranging from 0.5 to 25 μ
m
) and fixed concentrations (1 m
m
) of histone H3 peptide (residues 1–20). Rates of methylation are the means of duplicate measurements ± standard error of measurement. Apparent kinetic parameters were determined by fitting the data to the Michaelis-Menten equation (Equation 1, “Experimental Procedures”). B, comparison of WRA (open circles) and WRAD (open triangles) kinetics with histone H3 peptide as the variable substrate (ranging from 25 to 5000 μ
m
). The data are represented and fitted as described for A. For clarity, the values for the concentration range from 0 to 1000 μ
m
are shown. C, comparison of the enzymatic activity of WRAD (4.3 μ
m
) with increasing concentrations of AdoHyc (1–250 μ
m
). Activity assays were conducted with fixed concentrations of AdoMet (25 μ
m
) and histone H3 peptide (500 μ
m
). Each point represents the means ± S.E. of measurement from duplicate measurements. The data were fit to Equation 2 (“Experimental Procedures”).
FIGURE 4.
Zinc is required for the H3K4 methyltransferase activity of WRAD. A, WRAD enzymatic activity (mean ± S.E.) with increasing concentrations of EDTA. B, WRAD enzymatic activity (mean ± S.E.) with increasing concentrations of 1,10-phenanthroline. C, relative WRAD activity after preincubation with 1 m
m
EDTA and the addition of different divalent cations: zinc, cobalt, magnesium, or calcium. The data were normalized relative to the untreated control (0 m
m
EDTA) and represent the means ± the standard error of measurement from two independent experiments.
FIGURE 5.
MLL1 and WRAD are each required for methylation of nucleosomal histone H3 by the MLL1 core complex. A, comparison of the enzymatic activity of the MLL1 SET domain (M), WRA(D), or the fully assembled MLL1 core complex (MWRA) using the dialyzed histone octamer or reconstituted nucleosomes as substrates. Histone methyltransferase assays were conducted for a period of 8 h. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panels) and fluorography (lower panels, overnight (O/N) and 4-day exposures). B, mono-, di-, and trimethylation of reconstituted nucleosomes were compared using wild-type MLL3745 (M(wild-type)), WRAD, or the fully assembled MLL1 core complex (MWRAD). In addition, nucleosome methylation activities were compared among MLL1 core complexes assembled with a loss-of-function variant of MLL1 (M(N3906A)) or a gain-of-function variant of MLL1 (M(Y3942F)). Western blotting with antibodies specific for the H3K4 mono-, di-, or trimethylated forms of histone H3 was used to detect nucleosome methylation, as described under “Experimental Procedures.”
FIGURE 6.
Arginine 3765 of MLL1 is required for the interaction between MLL1 and WRAD and for nucleosomal histone H3 methylation. A, comparison of the enzymatic activity of wild-type (M) and R3765A (MR3765A) MLL1 SET domains in the presence and absence of WRAD using dialyzed histone octamers or reconstituted nucleosomes as substrates. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panel) and fluorography (3H-Methyl (lower panels), overnight (O/N) and 4-day exposures)). Lanes 1 and 2 are assays conducted with dialyzed histone octamers; lanes 3–7 are assays conducted with a reconstituted nucleosome substrate. B, diffusion-free sedimentation coefficient distribution (c(s)) derived from sedimentation velocity analytical ultracentrifugation of the MLL1 core complex assembled with wild type (upper panel) or the R3765A variant of MLL1 (lower panel). The experimental sedimentation coefficients (s) are indicated.
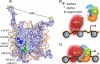
FIGURE 7.
Proposed model for the regulation of nucleosome methylation by the MLL1 core complex. A, surface model of the X. laevis nucleosome core particle (drawn with Protein Data Bank coordinate 1KX5 (70)). Highlighted are histone H2A and H2B residues required for H3K4 methylation in budding yeast (69). Histone H3K4 is highlighted in yellow, H2A residues are shown in dark blue, and H2B residues are green. B, MLL1 interacts with DNA through AT hooks and C_XX_C domains located in the MLL-N subunit of the MLL1 core complex. In the absence of WRAD, the histone H3 N-terminal tail interacts with linker DNA and is not a substrate for methylation. Consequently, transcription of MLL1 target genes is repressed. W, WDR5; R, RbBP5; A, Ash2L; and D, DPY-30. MLL-N is the 300-kDa N-terminal portion of MLL1, and MLL-C is the 180-kDa C-terminal portion of MLL1 containing the SET domain. C, the WDR5 component of WRAD interacts with Arg-3765 of MLL1 resulting in the assembly of the MLL1 core complex. The MLL1 complex interacts extensively with the nucleosome core and liberates the histone H3 N-terminal tail, which becomes a substrate for mono- and dimethylation at lysine 4, a critical step in the pathway that is required for transcription of MLL1-dependent genes (indicated by the dashed arrow).
Similar articles
- On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-1 (MLL1) core complex.
Patel A, Dharmarajan V, Vought VE, Cosgrove MS. Patel A, et al. J Biol Chem. 2009 Sep 4;284(36):24242-56. doi: 10.1074/jbc.M109.014498. Epub 2009 Jun 25. J Biol Chem. 2009. PMID: 19556245 Free PMC article. - An Ash2L/RbBP5 heterodimer stimulates the MLL1 methyltransferase activity through coordinated substrate interactions with the MLL1 SET domain.
Cao F, Chen Y, Cierpicki T, Liu Y, Basrur V, Lei M, Dou Y. Cao F, et al. PLoS One. 2010 Nov 23;5(11):e14102. doi: 10.1371/journal.pone.0014102. PLoS One. 2010. PMID: 21124902 Free PMC article. - Biochemical reconstitution and phylogenetic comparison of human SET1 family core complexes involved in histone methylation.
Shinsky SA, Monteith KE, Viggiano S, Cosgrove MS. Shinsky SA, et al. J Biol Chem. 2015 Mar 6;290(10):6361-75. doi: 10.1074/jbc.M114.627646. Epub 2015 Jan 5. J Biol Chem. 2015. PMID: 25561738 Free PMC article. - WRAD: enabler of the SET1-family of H3K4 methyltransferases.
Ernst P, Vakoc CR. Ernst P, et al. Brief Funct Genomics. 2012 May;11(3):217-26. doi: 10.1093/bfgp/els017. Epub 2012 May 30. Brief Funct Genomics. 2012. PMID: 22652693 Free PMC article. Review. - Diverse roles of WDR5-RbBP5-ASH2L-DPY30 (WRAD) complex in the functions of the SET1 histone methyltransferase family.
Ali A, Tyagi S. Ali A, et al. J Biosci. 2017 Mar;42(1):155-159. doi: 10.1007/s12038-017-9666-9. J Biosci. 2017. PMID: 28229975 Review.
Cited by
- Catalytic and functional roles of conserved amino acids in the SET domain of the S. cerevisiae lysine methyltransferase Set1.
Williamson K, Schneider V, Jordan RA, Mueller JE, Henderson Pozzi M, Bryk M. Williamson K, et al. PLoS One. 2013;8(3):e57974. doi: 10.1371/journal.pone.0057974. Epub 2013 Mar 1. PLoS One. 2013. PMID: 23469257 Free PMC article. - The role of Trithorax family regulating osteogenic and Chondrogenic differentiation in mesenchymal stem cells.
Ma Q, Song C, Yin B, Shi Y, Ye L. Ma Q, et al. Cell Prolif. 2022 May;55(5):e13233. doi: 10.1111/cpr.13233. Epub 2022 Apr 28. Cell Prolif. 2022. PMID: 35481717 Free PMC article. Review. - Substrate and product specificities of SET domain methyltransferases.
Del Rizzo PA, Trievel RC. Del Rizzo PA, et al. Epigenetics. 2011 Sep 1;6(9):1059-67. doi: 10.4161/epi.6.9.16069. Epub 2011 Sep 1. Epigenetics. 2011. PMID: 21847010 Free PMC article. Review. - Histone methyltransferases and demethylases: regulators in balancing osteogenic and adipogenic differentiation of mesenchymal stem cells.
Deng P, Chen QM, Hong C, Wang CY. Deng P, et al. Int J Oral Sci. 2015 Dec 18;7(4):197-204. doi: 10.1038/ijos.2015.41. Int J Oral Sci. 2015. PMID: 26674421 Free PMC article. Review. - Unraveling MLL1-fusion leukemia: Epigenetic revelations from an iPS cell point mutation.
Kobrossy L, Xu W, Zhang C, Feng W, Turner CE, Cosgrove MS. Kobrossy L, et al. J Biol Chem. 2024 Nov;300(11):107825. doi: 10.1016/j.jbc.2024.107825. Epub 2024 Sep 27. J Biol Chem. 2024. PMID: 39342993 Free PMC article.
References
- Santos-Rosa H., Schneider R., Bernstein B. E., Karabetsou N., Morillon A., Weise C., Schreiber S. L., Mellor J., Kouzarides T. (2003) Mol. Cell 12, 1325–1332 - PubMed
- Cosgrove M. S., Wolberger C. (2005) Biochem. Cell Biol. 83, 468–476 - PubMed
- Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. Y., Landry J., Kauer M., Tackett A. J., Chait B. T., Badenhorst P., Wu C., Allis C. D. (2006) Nature 442, 86–90 - PubMed
- Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002) Nature 419, 407–411 - PubMed
- Ruthenburg A. J., Allis C. D., Wysocka J. (2007) Mol. Cell 25, 15–30 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials