The growth cone cytoskeleton in axon outgrowth and guidance - PubMed (original) (raw)
Review
The growth cone cytoskeleton in axon outgrowth and guidance
Erik W Dent et al. Cold Spring Harb Perspect Biol. 2011.
Abstract
Axon outgrowth and guidance to the proper target requires the coordination of filamentous (F)-actin and microtubules (MTs), the dynamic cytoskeletal polymers that promote shape change and locomotion. Over the past two decades, our knowledge of the many guidance cues, receptors, and downstream signaling cascades involved in neuronal outgrowth and guidance has increased dramatically. Less is known, however, about how those cascades of information converge and direct appropriate remodeling and interaction of cytoskeletal polymers, the ultimate effectors of movement and guidance. During development, much of the communication that occurs between environmental guidance cues and the cytoskeleton takes place at the growing tip of the axon, the neuronal growth cone. Several articles on this topic focus on the "input" to the growth cone, the myriad of receptor types, and their corresponding cognate ligands. Others investigate the signaling cascades initiated by receptors and propagated by second messenger pathways (i.e., kinases, phosphatases, GTPases). Ultimately, this plethora of information converges on proteins that associate directly with the actin and microtubule cytoskeletons. The role of these cytoskeletal-associated proteins, as well as the cytoskeleton itself in axon outgrowth and guidance, is the subject of this article.
Figures
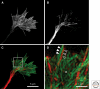
Figure 1.
F-actin and microtubule distribution in a hippocampal growth cone. (A) In this typical mouse hippocampal growth cone, labeled with fluorescent phalloidin, the F-actin is concentrated in filopodia (bundled F-actin) and lamellipodia (meshwork of F-actin), with relatively little F-actin in the axon shaft. (B) Microtubules, labeled with an antibody to tyrosinated tubulin, are concentrated as a bundle in the axon shaft but also splay apart in the growth cone, extending into distal peripheral regions. (C) A false-color overlay of images in A and B. Microtubules are in red and F-actin is in green. (D) A magnified view of the boxed region in C. Note the close apposition of an F-actin bundle (closed arrowheads) at the base of a filopodium and an individual microtubule (open arrowheads). At this magnification, the dendritic (D) actin meshwork can also be discerned.
Figure 2.
Structural characteristics of F-actin and actin-associated proteins. (A) Actin filaments are polar polymers composed of a barbed end, where the bulk of actin monomer addition occurs, and a pointed end, where dissociation of actin monomers occur. The nucleotide state of the actin changes as the filaments age (ATP→ADPpi→ADP). (B) F-actin in a filopodium forms bundles due to the action of bundling proteins. Actin monomers add onto existing filaments at the tip of the filopodium through the action of barbed-end binding proteins. Actin filaments are constantly undergoing retrograde flow (large vertical arrows) and are disassembled near their pointed ends by severing proteins. Motor proteins use the bundled F-actin to transport cargo both anterogradely and retrogradely. In contrast, F-actin in the lamellipodium forms a dendritic network through the action of dendritic nucleator proteins and capping proteins. Addition of actin monomers also occurs near the membrane, and disassembly occurs more proximally in the growth cone.
Figure 3.
Structural characteristics of microtubules and microtubule-associated proteins. (A) Microtubules are polar polymers composed of a plus end, where dimer addition and dissociation occur, and a minus end where dimer dissociation can occur. In neurons the minus end of microtubules is often stabilized. Microtubule dynamics occur primarily through polymerization and depolymerization at the plus end. The conversion of microtubule growth to shrinkage is termed “catastrophe” and the conversion from shrinkage to growth is termed “rescue” in this figure. The nucleotide state of tubulin also changes soon after dimer addition (GTP→GDP). (B) As microtubules polymerize, they bind +TIP proteins at their plus ends. There are many structural microtubule-associated proteins (MAPs) that usually act to stabilize the microtubule. Motor proteins, such as the kinesin family of proteins and cytoplasmic dynein, also transport cargos along microtubules. Several proteins aid in the depolymerization of microtubules, while others can sever microtubules.
Figure 4.
A working model of cytoskeletal dynamics in a growth cone exposed to a gradient of a positive guidance cue. (A) A schematic of a growth cone that includes actin bundles (green), an actin meshwork (blue), and microtubules (red). The guidance cue gradient (gray) is high in the upper right of the figure, toward which the growth cone is turning. Boxed regions of the growth cone are magnified in subsequent panels. (B) This region of the growth cone is undergoing protrusion. Protrusion is due to activation of barbed-end binding proteins and actin nucleators, resulting in protrusion of filopodia and lamellipodia, respectively. Actin severing can also occur, resulting in new barbed ends for growth. (C) This region of the growth cone is where the actin and microtubule cytoskeleton coordinate their activities, resulting in directed outgrowth. F-actin bundles can guide microtubules. The increased microtubule polymerization/stabilization on one side of the growth cone may favor polarized delivery of materials, which would subsequently favor growth in a particular direction. (D) This region of the growth cone is undergoing retraction. Actin bundles and dendritic networks are disassembled, potentially through the inactivation of barbed-end binding and actin bundling proteins, increased severing of F-actin without subsequent polymerization and continued myosin-driven retrograde actin flow. Microtubules may undergo increased catastrophe or decreased rescue, resulting in their departure from this side of the growth cone. Importantly, several of the cytoskeletal interactions outlined in this model are suggestions based on the current literature but have yet to be documented experimentally.
Similar articles
- Cytoskeletal dynamics and transport in growth cone motility and axon guidance.
Dent EW, Gertler FB. Dent EW, et al. Neuron. 2003 Oct 9;40(2):209-27. doi: 10.1016/s0896-6273(03)00633-0. Neuron. 2003. PMID: 14556705 Review. - Cytoskeletal social networking in the growth cone: How +TIPs mediate microtubule-actin cross-linking to drive axon outgrowth and guidance.
Cammarata GM, Bearce EA, Lowery LA. Cammarata GM, et al. Cytoskeleton (Hoboken). 2016 Sep;73(9):461-76. doi: 10.1002/cm.21272. Epub 2016 Feb 8. Cytoskeleton (Hoboken). 2016. PMID: 26783725 Free PMC article. Review. - Molecular mechanisms of axonal growth.
Bouquet C, Nothias F. Bouquet C, et al. Adv Exp Med Biol. 2007;621:1-16. doi: 10.1007/978-0-387-76715-4_1. Adv Exp Med Biol. 2007. PMID: 18269207 Review. - Axon guidance by growth cones and branches: common cytoskeletal and signaling mechanisms.
Dent EW, Tang F, Kalil K. Dent EW, et al. Neuroscientist. 2003 Oct;9(5):343-53. doi: 10.1177/1073858403252683. Neuroscientist. 2003. PMID: 14580119 Review. - Microtubules, actin and cytolinkers: how to connect cytoskeletons in the neuronal growth cone.
Pinto-Costa R, Sousa MM. Pinto-Costa R, et al. Neurosci Lett. 2021 Mar 16;747:135693. doi: 10.1016/j.neulet.2021.135693. Epub 2021 Jan 30. Neurosci Lett. 2021. PMID: 33529653
Cited by
- Reverse engineering of feedforward cortical-Hippocampal microcircuits for modelling neural network function and dysfunction.
Hanssen KS, Winter-Hjelm N, Niethammer SN, Kobro-Flatmoen A, Witter MP, Sandvig A, Sandvig I. Hanssen KS, et al. Sci Rep. 2024 Oct 29;14(1):26021. doi: 10.1038/s41598-024-77157-4. Sci Rep. 2024. PMID: 39472479 Free PMC article. - Murine Retina Outer Plexiform Layer Development and Transcriptome Analysis of Pre-Synapses in Photoreceptors.
Kim SY, Park CH, Moon BH, Seabold GK. Kim SY, et al. Life (Basel). 2024 Sep 2;14(9):1103. doi: 10.3390/life14091103. Life (Basel). 2024. PMID: 39337887 Free PMC article. - Neurons dispose of hyperactive kinesin into glial cells for clearance.
Xie C, Chen G, Li M, Huang P, Chen Z, Lei K, Li D, Wang Y, Cleetus A, Mohamed MA, Sonar P, Feng W, Ökten Z, Ou G. Xie C, et al. EMBO J. 2024 Jul;43(13):2606-2635. doi: 10.1038/s44318-024-00118-0. Epub 2024 May 28. EMBO J. 2024. PMID: 38806659 Free PMC article. - Morphogenetic theory of mental and cognitive disorders: the role of neurotrophic and guidance molecules.
Primak A, Bozov K, Rubina K, Dzhauari S, Neyfeld E, Illarionova M, Semina E, Sheleg D, Tkachuk V, Karagyaur M. Primak A, et al. Front Mol Neurosci. 2024 Apr 3;17:1361764. doi: 10.3389/fnmol.2024.1361764. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38646100 Free PMC article. Review. - Gut microbiota regulate maturation and mitochondrial function of the nutrient-sensing enteroendocrine cell.
Alsudayri A, Perelman S, Brewer M, Chura A, McDevitt M, Drerup C, Ye L. Alsudayri A, et al. Development. 2024 Apr 15;151(8):dev202544. doi: 10.1242/dev.202544. Epub 2024 Apr 30. Development. 2024. PMID: 38577841 Free PMC article.
References
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P 1995. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83: 269–278 - PubMed
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, et al. 2001. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci 4: 367–373 - PubMed
- Akhmanova A, Steinmetz MO 2008. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 9: 309–322 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM068678/GM/NIGMS NIH HHS/United States
- R01 NS064014-03/NS/NINDS NIH HHS/United States
- NS064014/NS/NINDS NIH HHS/United States
- R01 NS064014/NS/NINDS NIH HHS/United States
- R01 GM068678-08/GM/NIGMS NIH HHS/United States
- R01 GM068678/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous