The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k - PubMed (original) (raw)
The channel-kinase TRPM7 regulates phosphorylation of the translational factor eEF2 via eEF2-k
Anne-Laure Perraud et al. Cell Signal. 2011 Mar.
Abstract
Protein translation is an essential but energetically expensive process, which is carefully regulated in accordance to the cellular nutritional and energy status. Eukaryotic elongation factor 2 (eEF2) is a central regulation point since it mediates ribosomal translocation and can be inhibited by phosphorylation at Thr56. TRPM7 is the unique fusion of an ion channel with a functional Ser/Thr-kinase. While TRPM7's channel function has been implicated in regulating vertebrate Mg(2+) uptake required for cell growth, the function of its kinase domain remains unclear. Here, we show that under conditions where cell growth is limited by Mg(2+) availability, TRPM7 via its kinase mediates enhanced Thr56 phosphorylation of eEF2. TRPM7-kinase does not appear to directly phosphorylate eEF2, but rather to influence the amount of eEF2's cognate kinase eEF2-k, involving its phosphorylation at Ser77. These findings suggest that TRPM7's structural duality ensures ideal positioning of its kinase in close proximity to channel-mediated Mg(2+) uptake, allowing for the adjustment of protein translational rates to the availability of Mg(2+).
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
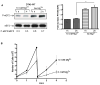
Figure 1. Endogenous eEF2 phosphorylation under growth-limiting Mg2+ deprivation in DT40 cells
(A) Analysis of eEF2 protein and P-Thr56 levels in wt DT40 cells by Western blot in response to reduced Mg2+-levels. DT40 cells were cultured in chemically defined medium with 10 mM MgCl2, spun down, resuspended and cultured in Mg2+-free medium for 1 or 2 h. The blot was probed with anti eEF2-P-Thr56, and reprobed with anti-eEF2. Densitometric quantification is underneath the corresponding lanes (arbitrary unit); values of two independent experiments plotted on the right (*P < 0.05). (B) Growth curves of DT40 wt cells cultured under the same set of conditions as in (A). n=3. Error bars represent the mean ± S.D.
Figure 2. Role of TRPM7 and its kinase domain in regulating native eEF2 phosphorylation upon Mg2+ deprivation
(A) Schematics of TRPM7 wt protein (TM=transmembrane span, CCR= Coiled Coil Region). (B) eEF2 protein and P-Thr56 levels in TRPM7−/− cells complemented with dox-inducible expression of hTRPM7 wt (cWT), phosphotransferase-deficient (cKR), and kinase-deleted (cΔK) mutants with 10 mM Mg2+. Blot probed with anti eEF2-P-Thr56, reprobed with anti-eEF2. Densitometric quantification underneath the corresponding lanes. (C) Levels of eEF2 protein and P-Thr56 in cWT vs. cΔK DT40s. DT40 cells were cultured in chemically defined medium with 10 mM MgCl2, spun down, and resuspended for 1 h in Mg2+-free medium. Western blot and densitometry as before. Normalized values of three independent experiments plotted. Mean value and SEM are indicated (*P < 0.05). (D) Comparison of cWT with cKR DT40s, using same culturing and Mg2+-starvation protocol as before. Western blot, densitometry and statistics as above; n=3 (*P < 0.05). (E) Flag-tagged eEF2 overexpressed in HEK-293 cells alone or with a C-terminal flag-tagged fragment of hTRPM7 containing the kinase (C3-M7, aa 1440-1865). In vitro phosphorylation assays in the presence of 100 μM Mg-ATP using anti-flag immunoprecipitates (25 min at 32°C). Analysis of eEF2-phosphorylation by blotting using anti P-Thr56 eEF2. Blot reprobed with anti-flag. Please note that the P-Thr56 eEF2 antibody recognizes phosphorylated C3-M7 fragment, which includes a Ser/Thr stretch found to be hyperphosphorylated on over 35 residues [44].
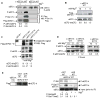
Figure 3. TRPM7 interacts with, and phosphorylates mouse eEF2-k at serine site 77
(A) Each 500 ng purified GST-eEF2-k (mouse) and MBP-hTRPM7-kinase (human) were mixed and immunoprecipitated with anti-eEF2-k. Co-associated TRPM7-kinase was detected by blotting with anti-MBP. n=2 (B) Left panel: Overexpression in HEK-293 cells of flag-eEF2-k alone, or with flag-TRPM7-kinase. In vitro phosphorylation assays using anti-flag immunoprecipitates with 10 mCi γ32P-ATP. Protein phosphorylation represented by autoradiography. Right panel: eEF2-k expressed alone or with a kinase-containing fragment of hTRPM7 (C3-M7, aa 1440–1865) in HEK-293 cells. In vitro phosphorylation assays with 100 μM Mg-ATP as above. Analysis of eEF2-k-phosphorylation by blotting using two different motif-specific anti phospho-serine antibodies. Protein expression levels verified with anti-flag. (C) Flag-eEF2-k wt expressed alone or with flag-TRPM7-C3 in HEK293 cells. Following flag-immunoprecipitation and in vitro phosphorylation, eEF2-k phosphorylation was analysed using anti P-Ser-77 eEF2-k (left panel). Similar experiment using the eEF2-k S77A mutant (right panel). Protein expression levels verified with anti-flag. The shown results are representative of three independent experiments.
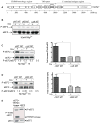
Figure 4. The increase in eEF2 Thr56-phosphorylation under hypomagnesic conditions requires eEF2-k
(A). Linear protein domain organization of eEF2-k, with the deleted catalytic region represented as a hatched box, and the N-terminal calmodulin (CaM) binding site as a black rectangle (upper drawing). Below, schematics of wt and mutated DT-40 eEF2-k alleles show the genomic region replaced by the puromycin and histidinol resistance cassettes. (B). Validation of the eEF2-k−/− DT40 cell line: RT-PCR experiments using cDNA obtained from the DT40 wt parental cell line, or from an eEF2-k−/− DT40 mutant clone were performed. Chicken eEF2-k specific primers were used to amplify fragments of endogenous DT40 eEF2-k, and primers against the house-keeping cNudT9 gene were used as a positive control (left panel). Lysates from 106 DT40 cells were generated in order to efficiently see basal eEF-2 Thr56 phosphorylation by immunoblotting using anti-P-Thr56-eEF2 antibody; the blot membrane was subsequently stripped and reprobed with anti-eEF2 antibody to assess protein loading levels. The results shown are representative of two separate experiments. (C). Mg2+-starving experiment was conducted as described previously (see Fig. 1), only that lysates were generated from 106 cells instead from 105 cells, to ensure we would be able to detect low signal intensities. DT40 eEF2-k−/− mutant cells and its complemented version (ceEF2-k) were cultured for 2 hours in complete, chemically defined media (serum-free) containing 0 or 10 mM MgCl2, and immunoblotting analysis of protein lysates from these cells was performed using anti-PThr56-eEF2 antibody. The blot membrane was subsequently stripped and reprobed with anti-eEF2. When appropriate, densitometric quantification is indicated underneath the corresponding lanes (arbitrary unit). The results shown are representative of three separate experiments.
Figure 5. The increase in eEF2 Thr56-phosphorylation under hypomagnesic conditions correlates with the amount of eEF2-k protein, which requires an intact eEF2-k Ser77 site
(A) Cell lysates from DT40s (eEF2-k KO line and its complemented version ceEF2-k wt) analysed by immuno-blot to detect eEF-2 and PThr56-eEF2 (top two lanes), as well as by flag-IP and subsequent blotting with anti-flag and anti P-Ser to represent protein amounts and Ser-phosphorylation of flag-tagged eEF2-k under varying Mg2+-conditions. (B) Cell lysates analysed by IP and blotting with anti-flag to represent protein amounts of flag-tagged eEF2-k in DT40 cells cultured for 2 hours in complete, chemically defined media with high Mg2+ (10mM), standard (1mM), or low (close to 0). Levels of eEF2 in the lysates assessed as control; representative of two separate experiments (C). Flag-eEF2-k protein levels and eEF2 basal phosphorylation in eEF2-k−/− DT40 cells complemented with eEF2-k wt, or mutants S77A and S77D, under standard physiological Mg2+ (1mM). Equal numbers of ceEF2-k wt, cS77A and cS77D DT40s were cultured with dox and 1mM Mg2+. Densitometric quantification is underneath the corresponding lanes. Representative of two separate experiments (D) Similar experiment as in (C), but under varying Mg2+ conditions (as indicated). (E) Left panel, stable, dox-inducible protein-expression of flag-tagged mouse eEF2-k wt in TRPM7−/− DT40 cells complemented with hTRPM7 WT or KR-mutant. Right panel, Analysis of eEF2-k phosphorylation in these cell lines: Equal numbers of cells (2×107) stably co-expressing flag-eEF2-k and either TRPM7 WT or TRPM7 KR in the TRPM7−/−background were cultured with dox under different Mg2+ conditions. Cell lysates analysed by flag-IP and blotting. Blot reprobed with anti-flag. Densitometric quantification is underneath the corresponding lanes. Unless stated otherwise, shown experiments all representative of three independent experiments.
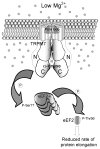
Figure 6. Model of TRPM7’s involvement in eEF2-k/eEF2 regulation in response to suboptimal extracellular Mg2+-levels
A vertical slice through the plasma membrane is showing “half-a-pore” of the TRPM7 channel kinase, as the complete channel is thought to be tetrameric. Both TRPM7 protein termini, including the C-terminal kinase are cytosolic. “P” indicates phosphorylation.
Similar articles
- Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation.
Kaul G, Pattan G, Rafeequi T. Kaul G, et al. Cell Biochem Funct. 2011 Apr;29(3):227-34. doi: 10.1002/cbf.1740. Epub 2011 Mar 10. Cell Biochem Funct. 2011. PMID: 21394738 Review. - Phosphorylation of eukaryotic elongation factor 2 (eEF2) by cyclin A-cyclin-dependent kinase 2 regulates its inhibition by eEF2 kinase.
Hizli AA, Chi Y, Swanger J, Carter JH, Liao Y, Welcker M, Ryazanov AG, Clurman BE. Hizli AA, et al. Mol Cell Biol. 2013 Feb;33(3):596-604. doi: 10.1128/MCB.01270-12. Epub 2012 Nov 26. Mol Cell Biol. 2013. PMID: 23184662 Free PMC article. - Cyclic AMP inhibits translation of cyclin D3 in T lymphocytes at the level of elongation by inducing eEF2-phosphorylation.
Gutzkow KB, Låhne HU, Naderi S, Torgersen KM, Skålhegg B, Koketsu M, Uehara Y, Blomhoff HK. Gutzkow KB, et al. Cell Signal. 2003 Sep;15(9):871-81. doi: 10.1016/s0898-6568(03)00038-x. Cell Signal. 2003. PMID: 12834812 - The channel-kinase TRPM7, revealing the untold story of Mg(2+) in cellular signaling.
Schmitz C, Brandao K, Perraud AL. Schmitz C, et al. Magnes Res. 2014 Jan-Mar;27(1):9-15. doi: 10.1684/mrh.2014.0357. Magnes Res. 2014. PMID: 24752033 Review. - TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions.
Brandao K, Deason-Towne F, Zhao X, Perraud AL, Schmitz C. Brandao K, et al. Cell Mol Life Sci. 2014 Dec;71(24):4853-67. doi: 10.1007/s00018-014-1647-7. Epub 2014 May 25. Cell Mol Life Sci. 2014. PMID: 24858416 Free PMC article.
Cited by
- Mibefradil represents a new class of benzimidazole TRPM7 channel agonists.
Schäfer S, Ferioli S, Hofmann T, Zierler S, Gudermann T, Chubanov V. Schäfer S, et al. Pflugers Arch. 2016 Apr;468(4):623-34. doi: 10.1007/s00424-015-1772-7. Epub 2015 Dec 16. Pflugers Arch. 2016. PMID: 26669310 - Inactivation of TRPM7 kinase activity does not impair its channel function in mice.
Kaitsuka T, Katagiri C, Beesetty P, Nakamura K, Hourani S, Tomizawa K, Kozak JA, Matsushita M. Kaitsuka T, et al. Sci Rep. 2014 Jul 17;4:5718. doi: 10.1038/srep05718. Sci Rep. 2014. PMID: 25030553 Free PMC article. - Transient Receptor Potential Melastatin 7 Cation Channel Kinase: New Player in Angiotensin II-Induced Hypertension.
Antunes TT, Callera GE, He Y, Yogi A, Ryazanov AG, Ryazanova LV, Zhai A, Stewart DJ, Shrier A, Touyz RM. Antunes TT, et al. Hypertension. 2016 Apr;67(4):763-73. doi: 10.1161/HYPERTENSIONAHA.115.07021. Epub 2016 Feb 29. Hypertension. 2016. PMID: 26928801 Free PMC article. - TRPM channels in health and disease.
Chubanov V, Köttgen M, Touyz RM, Gudermann T. Chubanov V, et al. Nat Rev Nephrol. 2024 Mar;20(3):175-187. doi: 10.1038/s41581-023-00777-y. Epub 2023 Oct 18. Nat Rev Nephrol. 2024. PMID: 37853091 Review. - Magnesium Deficiency Causes a Reversible, Metabolic, Diastolic Cardiomyopathy.
Liu M, Liu H, Feng F, Xie A, Kang GJ, Zhao Y, Hou CR, Zhou X, Dudley SC Jr. Liu M, et al. J Am Heart Assoc. 2021 Jun 15;10(12):e020205. doi: 10.1161/JAHA.120.020205. Epub 2021 Jun 5. J Am Heart Assoc. 2021. PMID: 34096318 Free PMC article.
References
- Ryazanov AG, Shestakova EA, Natapov PG. Nature. 1988;334(6178):170–173. - PubMed
- Proud CG. Curr Top Microbiol Immunol. 2004;279:215–244. - PubMed
- Ryazanov AG. FEBS Lett. 2002;514(1):26–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08AI060926/AI/NIAID NIH HHS/United States
- R01 GM090123/GM/NIGMS NIH HHS/United States
- R01GM057300/GM/NIGMS NIH HHS/United States
- K08 AI060926/AI/NIAID NIH HHS/United States
- R01GM068801/GM/NIGMS NIH HHS/United States
- R01 GM068801/GM/NIGMS NIH HHS/United States
- R01GM90123/GM/NIGMS NIH HHS/United States
- R01 GM057300/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous