Nasal potential difference to detect Na+ channel dysfunction in acute lung injury - PubMed (original) (raw)
Review
Nasal potential difference to detect Na+ channel dysfunction in acute lung injury
R Mac Sweeney et al. Am J Physiol Lung Cell Mol Physiol. 2011 Mar.
Abstract
Pulmonary fluid clearance is regulated by the active transport of Na(+) and Cl(-) through respiratory epithelial ion channels. Ion channel dysfunction contributes to the pathogenesis of various pulmonary fluid disorders including high-altitude pulmonary edema (HAPE) and neonatal respiratory distress syndrome (RDS). Nasal potential difference (NPD) measurement allows an in vivo investigation of the functionality of these channels. This technique has been used for the diagnosis of cystic fibrosis, the archetypal respiratory ion channel disorder, for over a quarter of a century. NPD measurements in HAPE and RDS suggest constitutive and acquired dysfunction of respiratory epithelial Na(+) channels. Acute lung injury (ALI) is characterized by pulmonary edema due to alveolar epithelial-interstitial-endothelial injury. NPD measurement may enable identification of critically ill ALI patients with a susceptible phenotype of dysfunctional respiratory Na(+) channels and allow targeted therapy toward Na(+) channel function.
Figures
Fig. 1.
Schematic diagram of alveolar type 1 and 2 cells showing distribution of ion channels, pumps, aquaporins (AQP), and β-receptors (see text, under Mechanism of alveolar Na + transport and fluid absorption, for explanations of circled numbers in figure). HSC, highly selective cation channel; PSC, poorly selective cation channel; NSC, nonselective cation channel; CNG, cyclic nucleotide-gated channel; CFTR, cystic fibrosis transmembrane conductance regulator.
Fig. 2.
Measurement of nasal potential difference (NPD).
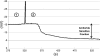
Fig. 3.
Typical NPD tracing from a healthy volunteer (see text for details). 1 = Perfusion with Ringers solution; 2 = perfusion with amiloride solution; vertical line indicates onset of amiloride perfusion.
Fig. 4.
Schematic representation of respiratory potential differences with associated height of respiratory liquid layers. Data adapted from Refs. , , , , .
Similar articles
- Systematic review and meta-analysis of nasal potential difference in hypoxia-induced lung injury.
Su Z, Zhu L, Wu J, Zhao R, Ji HL. Su Z, et al. Sci Rep. 2016 Aug 4;6:30780. doi: 10.1038/srep30780. Sci Rep. 2016. PMID: 27488696 Free PMC article. Review. - Specialized Pro-resolving Mediators Regulate Alveolar Fluid Clearance during Acute Respiratory Distress Syndrome.
Wang Q, Yan SF, Hao Y, Jin SW. Wang Q, et al. Chin Med J (Engl). 2018 Apr 20;131(8):982-989. doi: 10.4103/0366-6999.229890. Chin Med J (Engl). 2018. PMID: 29664060 Free PMC article. Review. - Adenosine A2B receptor activation stimulates alveolar fluid clearance through alveolar epithelial sodium channel via cAMP pathway in endotoxin-induced lung injury.
Wang M, Guo X, Zhao H, Lv J, Wang H, An Y. Wang M, et al. Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L787-L800. doi: 10.1152/ajplung.00195.2019. Epub 2020 Mar 4. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32129084 - Does High Alveolar Fluid Reabsorption Prevent HAPE in Individuals with Exaggerated Pulmonary Hypertension in Hypoxia?
Betz T, Dehnert C, Bärtsch P, Schommer K, Mairbäurl H. Betz T, et al. High Alt Med Biol. 2015 Dec;16(4):283-9. doi: 10.1089/ham.2015.0050. Epub 2015 Aug 10. High Alt Med Biol. 2015. PMID: 26258866 - Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid.
Fisher BJ, Kraskauskas D, Martin EJ, Farkas D, Wegelin JA, Brophy D, Ward KR, Voelkel NF, Fowler AA 3rd, Natarajan R. Fisher BJ, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L20-32. doi: 10.1152/ajplung.00300.2011. Epub 2012 Apr 20. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22523283
Cited by
- Aquaporine-5 and epithelial sodium channel β-subunit gene expression in gastric aspirates in human term newborns.
Castorena-Torres F, Alcorta-García MR, Lara-Díaz VJ. Castorena-Torres F, et al. Heliyon. 2018 Apr 13;4(4):e00602. doi: 10.1016/j.heliyon.2018.e00602. eCollection 2018 Apr. Heliyon. 2018. PMID: 29862364 Free PMC article. - Systematic review and meta-analysis of nasal potential difference in hypoxia-induced lung injury.
Su Z, Zhu L, Wu J, Zhao R, Ji HL. Su Z, et al. Sci Rep. 2016 Aug 4;6:30780. doi: 10.1038/srep30780. Sci Rep. 2016. PMID: 27488696 Free PMC article. Review. - δ ENaC: a novel divergent amiloride-inhibitable sodium channel.
Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. Ji HL, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Dec 15;303(12):L1013-26. doi: 10.1152/ajplung.00206.2012. Epub 2012 Sep 14. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22983350 Free PMC article. Review.
References
- Agarwal R, Srinivas R, Nath A, Jindal SK. Is the mortality higher in the pulmonary vs the extrapulmonary ARDS? Chest 133: 1463–1473, 2008. - PubMed
- Ahrens RC, Standaert TA, Launspach J, Han SH, Teresi ME, Aitken ML, Kelley TJ, Hilliard KA, Milgram LJH, Konstan MW, Weatherly MR, McCarty NA. Use of nasal potential difference and sweat chloride as outcome measures in multicenter clinical trials in subjects with cystic fibrosis. Pediatr Pulmonol 33: 142–150, 2002 - PubMed
- Alton EW, Currie D, Logan-Sinclair R, Warner JO, Hodson ME, Geddes DM. Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Respir J 3: 922–926, 1990 - PubMed
- Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, Pinsky MR. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1389–1394, 2001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources