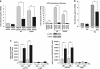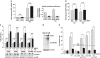Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress - PubMed (original) (raw)
doi: 10.1038/cdd.2010.142. Epub 2010 Nov 26.
A Schlierf, A K Lutz, J Shan, A Deinlein, J Kast, Z Galehdar, V Palmisano, N Patenge, D Berg, T Gasser, R Augustin, D Trümbach, I Irrcher, D S Park, W Wurst, M S Kilberg, J Tatzelt, K F Winklhofer
Affiliations
- PMID: 21113145
- PMCID: PMC3131924
- DOI: 10.1038/cdd.2010.142
Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress
L Bouman et al. Cell Death Differ. 2011 May.
Abstract
Loss of parkin function is responsible for the majority of autosomal recessive parkinsonism. Here, we show that parkin is not only a stress-protective, but also a stress-inducible protein. Both mitochondrial and endoplasmic reticulum (ER) stress induce an increase in parkin-specific mRNA and protein levels. The stress-induced upregulation of parkin is mediated by ATF4, a transcription factor of the unfolded protein response (UPR) that binds to a specific CREB/ATF site within the parkin promoter. Interestingly, c-Jun can bind to the same site, but acts as a transcriptional repressor of parkin gene expression. We also present evidence that mitochondrial damage can induce ER stress, leading to the activation of the UPR, and thereby to an upregulation of parkin expression. Vice versa, ER stress results in mitochondrial damage, which can be prevented by parkin. Notably, the activity of parkin to protect cells from stress-induced cell death is independent of the proteasome, indicating that proteasomal degradation of parkin substrates cannot explain the cytoprotective activity of parkin. Our study supports the notion that parkin has a role in the interorganellar crosstalk between the ER and mitochondria to promote cell survival under stress, suggesting that both ER and mitochondrial stress can contribute to the pathogenesis of Parkinson's disease.
Figures
Figure 1
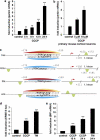
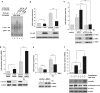
Mitochondrial stress induced by CCCP activates the UPR and leads to an upregulation of parkin. (a) Parkin mRNA levels are increased in response to mitochondrial membrane dissipation, induced by CCCP. SH-SY5Y cells were incubated with 10 _μ_M CCCP for the indicated time. Cells were collected and total cellular RNA was isolated and subjected to quantitative RT-PCR using parkin-specific primers. The amount of RNA of each sample was normalized with respect to the endogenous housekeeping gene _β_-actin. Shown is the fold increase of parkin-specific mRNA compared with untreated control cells. (b) Parkin mRNA is upregulated upon CCCP treatment in primary mouse cortical neurons. Primary cortical neurons derived from embryonic mouse brain were incubated with CCCP (10 _μ_M) for 12 h and analyzed as described in (a). (c) Human, mouse, bovine and equine promoter sequences of parkin, which are elongated downstream of the transcription start site (TSS) by 150 bp. Red arrow indicates the TSS and positions are denoted with relative to the TSS. The CREB/ATF-binding sites are indicated by semicircles. Red, yellow and blue semicircles are predicted by three different binding motifs, which correspond to a Genomatix-defined family of 14 matrices describing the CREB/ATF-binding site. The red and yellow colored binding sites are conserved between Homo sapiens, Bos taurus and Equus caballus, and H. sapiens and E. caballus, respectively, whereas the blue binding site is conserved across all four species. The green semicircles (not conserved) are additional binding sites. Downstream of the TSS, in the first intron of the parkin gene, an additional CREB/ATF-binding site is located in H. sapiens, Mus musculus and B. taurus. The consensus ATF4-binding site is written in bold letters. hsa, Homo sapiens; mmu, Mus musculus; bta, Bos taurus; eca, Equus caballus. (d and e) CCCP activates the UPR and causes ER stress. (d) The ER stress luciferase reporter construct ER stress-response element II (ERSE-II-luc) is activated by CCCP. HEK293T cells were transfected with the ERSE-II-luc reporter. At 24 h after transfection, the cells were treated with 10 _μ_M CCCP for 24 h. As a positive control, the cells were treated with the ER stressor tunicamycin (2 _μ_g/ml, 24 h). Shown is the fold induction of luciferase activity in CCCP-treated cells in comparison with non-treated control cells. Quantification is based on triplicates of at least three independent experiments. (e) BiP expression is increased in response to CCCP treatment. As an indicator of ER stress, BiP mRNA levels were analyzed in SH-SY5Y cells treated with CCCP (10 _μ_M) for the indicated time by quantitative RT-PCR as described in Figure 1a. Tunicamycin (2 _μ_g/ml) was used as a positive control to induce ER stress. ***P<0.001, **P<0.01
Figure 2
Parkin gene expression is upregulated in response to ER stress. (a and b) Parkin mRNA levels are increased under ER stress induced by thapsigargin or tunicamycin. SH-SY5Y cells were incubated with 1 _μ_M thapsigargin (TG) (a) or 2 _μ_g/ml tunicamycin (TM) (b) for the indicated time. Cells were collected and total cellular RNA was isolated and subjected to quantitative RT-PCR using parkin-specific primers. The amount of RNA of each sample was normalized with respect to the endogenous housekeeping gene _β_-actin. The same results were obtained when 18sRNA was used as a control gene (data not shown). Shown is the fold increase of parkin-specific mRNA compared with untreated control cells. (c) Amino acid starvation leads to an upregulation of parkin mRNA. SH-SY5Y cells were treated with 2 mM -histidinol in cell culture medium, containing 10% dialysed FCS, for 14 h. The cells were then collected and total cellular RNA was isolated and subjected to quantitative RT-PCR using parkin-specific primers as described under Figure 1a. (d–f) Parkin protein expression is increased after ER stress induced by TG, TM, or amino acid starvation. Expression of endogenous parkin after treatment of SH-SY5Y cells with TG (d), TM (e) or -histidinol (f) for 14 h was analyzed by western blotting using the anti-parkin mAb PRK8. Loading was controlled by re-probing the blots for _β_-actin. The western blot image (e) was re-arranged by excluding one line, as indicated by a white line; all samples originate from one gel. (g–i) Parkin mRNA is upregulated on ER stress in HEK293T cells, mouse embryonic fibroblasts and primary mouse cortical neurons. HEK293 T cells (g), mouse embryonic fibroblasts (h), or primary cortical neurons derived from embryonic mouse brain (i) were incubated with TG (1 _μ_M) or TM (2 _μ_g/ml; primary cortical neurons: 3 _μ_g/ml) for 12 or 8 h and 12 h (primary cortical neurons) and analyzed as described in (a). ***P<0.001, **P<0.01, *P<0.05
Figure 3
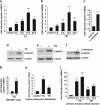
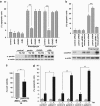
Transcriptional upregulation of parkin under ER stress is mediated by ATF4. (a) Schematic representation of the consensus ATF4-binding site, the putative ATF4-binding site within the parkin promoter and the luciferase reporter constructs cloned for the analysis described in the following: park-luc contains the putative ATF4-binding site of the parkin promoter in triplicate, mutant park-luc habors two point mutations within the putative ATF4-binding site, and ATF4RE-luc contains the confirmed ATF4-binding site of the insulin growth factor binding protein 1 (IGFBP1) promoter in triplicate. Of note, the putative binding site for ATF4 within the parkin promoter is located on the complementary strand in 5′ → 3′ direction. (b) The park-luc reporter construct is induced after ER stress. HEK293 T cells or SH-SY5Y cells were transfected with either the control luciferase reporter construct pGL3-luc (vector), the ATF4RE-luc construct containing the confirmed ATF4-binding site, the park-luc construct or the park-luc construct with a mutated ATF4 binding site (mut. park). At 8 h after transfection, cells were incubated with 1 _μ_M thapsigargin (TG) and collected after 14 h of treatment. Shown is the fold induction of luciferase activity in stressed cells compared with the non-stressed control based on triplicates of at least three independent experiments. (c) Increased expression of ATF4 or upstream PERK induces transcription from the park-luc reporter construct. HEK293T cells were co-transfected with the ATF4RE-luc reporter plasmid or the park-luc reporter plasmid and ATF4, PERK or GFP (as a control). As a positive control, one set of cells was treated with TG as described under (b) Shown is the fold induction of luciferase activity compared with GFP-expressing control cells based on triplicates of at least three independent experiments (left panel). Expression levels of ATF4 and PERK were analyzed by immunoblotting using the anti-ATF4 pAb C-20 or the anti-myc mAb 9E10 (right panels). Notably, TG treatment (1 _μ_M, 14 h) induced the increased expression of endogenous ATF4. Loading was controlled by re-probing the blots for _β_-actin. (d) A dominant-negative mutant of ATF4 (ATF4ΔN) interferes with the activation of the park-luc reporter construct in response to ER stress. HEK293T cells were co-transfected with the park-luc reporter plasmid and ATF4, ATF4ΔN, or GFP (as a control). At 8 h after transfection, cells were incubated with 1 _μ_M TG for 14 h. Shown is the fold induction of luciferase activity in comparison with GFP-expressing control cells based on triplicates of at least three independent experiments (left panel). Expression levels of ATF4 and ATF4ΔN were analyzed by immunoblotting using the anti-ATF4 pAb C-20 (right panel). Loading was controlled by re-probing the blots for _β_-actin. ***P<0.001, **P<0.01, n.s.=not significant
Figure 4
ATF4 binds to the parkin promoter and mediates parkin upregulation in response to ER and mitochondrial stress. (a) ER and mitochondrial stress-induced upregulation of parkin is impaired in ATF4-deficient cells. SH-SY5Y cells were transfected with ATF4-specific or control siRNA duplexes. Two days later, cells were re-transfected with siRNA duplexes and then incubated with 1 _μ_M TG or 10 _μ_M CCCP for 14 h. The cells were collected and analyzed as described in Figure 1a by quantitative RT-PCR using parkin-specific or ATF4-specific primers. The amount of RNA of each sample was normalized with respect to _β_-actin. Shown is the fold increase of parkin mRNA in response to TG or CCCP treatment (left panel). The efficiency of ATF4 downregulation was determined by quantitative RT-PCR (right panel) and western blotting (lower right panel) using the anti-ATF4 pAb C-20. (b) ER stress-induced upregulation of parkin is impaired in primary cortical neurons from ATF4-knockout mice. Primary cortical neurons from ATF4-deficient or wild-type mice were treated with tunicamycin (3 _μ_g/ml) for 8 h. Total RNA was isolated and analyzed using parkin-specific primers as described in Figure 1a. (c) ATF4 binds to the parkin promoter in vivo. HEK293T cells or SH-SY5Y cells incubated with or without 300 nM TG for 2 and 8 h were used to perform a ChIP analysis using a pAb specific for ATF4 in comparison with a nonspecific rabbit IgG. For the final real-time PCR step, primers specific for the parkin promoter region were used. ***P<0.001, **P<0.01, *P<0.05
Figure 5
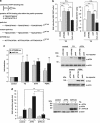
c-Jun suppresses the upregulation of parkin after ER stress. (a) c-Jun binds to the parkin oligonucleotide harboring the CREB/ATF-binding site. HEK293 T cells were incubated with 2 _μ_M thapsigargin (TG) and collected after 3 h. Nuclear extracts were prepared and tested for binding to the 32P-labeled oligonucleotide comprising the putative ATF4-binding site within the parkin promoter (park oligo; lanes 1–5) by an electrophoretic mobility shift assay (EMSA). The labeled oligonucleotides were incubated with nuclear extracts in the absence or presence of a 100-fold excess of unlabeled park oligo (lane 5) to compete with the binding reaction. To test for supershift activity, the anti-c-Jun pAb (N) sc-45X (lane 3) or the anti-ATF4 pAb C-20 (lane 4) was added to the binding reaction. (b) c-Jun decreases transcription from the park-luc reporter after ER stress. HEK293 T cells were co-transfected with the park-luc reporter construct and c-Jun or GFP (as a control). Eight hours after transfection, the cells were treated with 1 _μ_M TG for 14 h. Shown is the fold induction of luciferase activity in c-Jun-expressing cells in comparison with GFP-expressing control cells based on triplicates of at least three independent experiments. Expression levels of c-Jun were analyzed by immunoblotting using the anti-c-Jun pAb (N) sc-45 (lower panel). Protein (3 _μ_g) of total cell lysates was loaded. Loading was controlled by re-probing the blots for _β_-actin. (c) c-Jun suppresses the ATF4-mediated activation of the park-luc construct. HEK293 T cells were co-transfected with the park-luc reporter plasmid and either GFP (as a control), ATF4 plus GFP or ATF4 plus c-Jun. Eight hours after transfection, the cells were treated with 1 _μ_M TG for 14 h. Shown is the fold induction of luciferase activity in ATF4-expressing cells in comparison with ATF4- and c-Jun-expressing cells based on triplicates of at least three independent experiments. Expression levels of ATF4 and c-Jun were analyzed by immunoblotting using the anti-ATF4 pAb C-20 or the anti-c-Jun pAb (N) sc-45. Protein (3 _μ_g) of total cell lysates was loaded. Loading was controlled by re-probing the blots for _β_-actin (lower panel). (d) ER stress-induced upregulation of parkin is increased in c-Jun-deficient cells. SH-SY5Y cells were transfected with c-Jun-specific or control siRNA duplexes. One day later, cells were re-transfected with siRNA duplexes and incubated with 1 _μ_M TG for 14 h. The cells were collected and analyzed as described in Figure 1a by quantitative RT-PCR using parkin-specific primers. The amount of RNA of each sample was normalized with respect to _β_-actin. Shown is the fold increase of parkin mRNA in response to TG treatment (upper panel). The efficiency of c-Jun downregulation was determined by western blotting using the anti-c-Jun anti-c-Jun pAb (N) sc-45 (lower panel). Protein (30 _μ_g) of total cell lysates was loaded. (e) JNK3 decreases transcription from the park-luc reporter. HEK293 T cells were co-transfected with the park-luc reporter plasmid and JNK3 or GFP (as a control). Twenty four hours after transfection, the cells were treated with 1 _μ_M TG for 8 h. Shown is the fold induction of luciferase activity in JNK3-expressing cells in comparison with GFP-expressing control cells based on triplicates of at least three independent experiments. Expression levels of JNK3 were analyzed by immunoblotting using an anti-JNK pAB (lower panel). Loading was controlled by re-probing the blots for _β_-actin. (f) The JNK inhibitor SP600125 increases parkin upregulation in response to ER stress. SH-SY5Y cells were treated with or without the JNK inhibitor SP600125 (10 _μ_M) for 24 h. Thapsigargin was added after 10 h for additional 14 h. To quantify parkin-specific mRNA, cells were collected and analyzed as described in Figure 1a for quantitative RT-PCR using parkin-specific primers. The amount of RNA of each sample was normalized with respect to _β_-actin. Shown is the fold increase of parkin mRNA. Parkin protein levels were analyzed by immunoblotting using an anti-parkin PRK8 mAb (lower panel). The efficiency of SP600125 was controlled by blotting against phosphorylated c-Jun using the phospho-specific anti-c-Jun antibody X (Ser63) II pAb. Protein (15 _μ_g) of total cell lysates was loaded. Loading was controlled by re-probing the blots for c-Jun and _β_-actin. ***P<0.001, **P<0.01, *P<0.05
Figure 6
Parkin protects cells from ER stress-induced cell death. (a) Increased expression of wild-type (wt) parkin protects cells from ER stress-induced cell death. SH-SY5Y cells were co-transfected with EYFP (as a control) and wt parkin or the pathogenic parkin mutants G430D or ΔUBL. Twenty four hours after transfection, cells were incubated with 10 _μ_M thapsigargin (TG) or 5 _μ_g/ml tunicamycin (TM) at 37 °C for 8 h, fixed, permeabilized, and then the activation of caspase-3 was analyzed by indirect immunofluorescence using an anti-active caspase-3 pAb. Shown is the percentage of apoptotic cells among transfected cells. Parkin expression levels were determined by immunoblotting using the anti-parkin PRK8 mAb. Loading was controlled by re-probing the blots for _β_-actin (lower panel). (b) Parkin-deficient cells are more vulnerable to ER stress-induced cell death. SH-SY5Y cells were transfected with parkin-specific or control siRNA duplexes and co-transfected with EYFP (as a control) or siRNA-resistant wt parkin (rescue parkin). Three days later, the cells were stressed with TG (10 _μ_M) for 8 h fixed, permeabilized, and then the activation of caspase-3 was analyzed by indirect immunofluorescence as described in A. Parkin expression levels were determined by immunoblotting using the anti-parkin PRK8 mAb. Loading was controlled by re-probing the blots for _β_-actin (lower panel). (c) Mouse embryonic fibroblasts (MEFs) derived from parkin-knockout mice are more vulnerable to ER stress than wt MEFs. MEFs from wt or parkin-knockout (ko) mice were stressed with TG (10 _μ_M) for 16 h and then cellular viability was determined by the MTT assay. Shown is the relative viability of ko MEFs in comparison with wt MEFs after TG treatment. Quantification is based on five independent experiments. (d) Skin fibroblasts of patients carrying pathogenic mutations in the parkin gene are more vulnerable to ER stress. Skin fibroblasts from patients and control indivduals were stressed with tunicamycin (TM, 10 _μ_M) for 24 h, fixed, permeabilized, and then the activation of caspase-3 was analyzed by indirect immunofluorescence as described in (a). ***P< 0.001, **P<0.01, *P<0.05
Figure 7
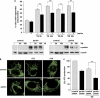
Parkin has no direct effect on ER stress. (a) Parkin deficiency does not increase the level of ER stress. SH-SY5Y cells were transfected with parkin-specific or control siRNA duplexes. Three days later, the cells were stressed with 1 _μ_M thapsigargin (TG) for 5 h. As an indicator of ER stress BiP mRNA levels were analyzed by quantitative RT-PCR as described in Figure 1a (left panel). To test for the efficiency of parkin knockdown, parkin mRNA levels were quantified in parallel (right panel). (b) The level of ER stress is not increased in mouse embryonic fibroblasts (MEFs) derived from parkin-knockout mice. MEFs from wildtype (wt) or parkin-knockout (ko) mice were stressed with 1 _μ_M TG or 2 _μ_g/ml tunicamycin (TM) for 5 h. The levels of BiP mRNA were analyzed by RT-PCR as described in Figure 1a. (c) Overexpression of parkin has no influence on the ER stress level determined by ER stress reporter constructs. HEK293 T cells were co-transfected with the ER stress reporter plasmids indicated (Supplementary Figure 5) and either parkin or GFP (as a control). Twenty four hours after transfection, the cells were treated with 1 _μ_M TG for 8 h. Shown is the fold induction of luciferase activity in parkin-expressing cells in comparison with GFP-expressing control cells. Quantification is based on triplicates of at least three independent experiments. Expression levels of parkin were analyzed by immunoblotting using the anti-parkin pAb 2132 (lower panel). Loading was controlled by re-probing the blots for _β_-actin. (d and e) The protective activity of parkin after ER stress is independent of the proteasome. (d) The efficiency of proteasomal inhibition by epoxomycin was demonstrated by an accumulation of endogenous p53 and ubiquitylated proteins. For immunoblotting, an anti-p53 and anti-ubiquitin mAb was used. Loading was controlled by re-probing the blots for _β_-actin. (e) Proteasomal inhibition does not impair the protective activity of parkin. SH-SY5Y cells were cotransfected with EYFP (as a control) or wild-type parkin. Twenty four hours after transfection, cells were incubated with 10 _μ_M thapsigargin (TG) and/or epoxomycin (epox, 0.1 or 10 _μ_M ) for 8 h, fixed, permeabilized, and then activation of caspase-3 was analyzed as described in Figure 6a. ***P<0.001, **P<0.01, n.s.=not significant
Figure 8
Parkin prevents mitochondrial damage and dysfunction induced by ER stress. (a and b) Increased parkin expression suppresses ER stress-induced mitochondrial fragmentation. SH-SY5Y cells were transfected with parkin or mCherry (as a control). One day after transfection, the cells were treated with thapsigargin (TG, 1 _μ_M for 5 or 16 h) or tunicamycin (TM, 2 _μ_g/ml for 5 or 16 h). Mitochondria were visualized by life cell microscopy after incubating cells with the fluorescent dye DiOC6(3). (a) The mitochondrial morphology was classified as tubular or fragmented (small rod-like or spherical mitochondria). Shown is the percentage of cells with fragmented mitochondria. Quantifications were based on triplicates of three independent experiments. For each experiment ⩾300 cells per coverslip of triplicate samples were assessed. Expression levels of parkin were analyzed by immunoblotting using the anti-parkin pAb 2132 (lower panel). Loading was controlled by re-probing the blots for _β_-actin. (b) Examples of mitochondrial morphologies of the experiment described in (a). Treatment of cells with TG or TM cause a disruption of the tubular mitochondrial network, which can be suppressed by increased parkin expression. (c) Parkin deficiency increases ATP depletion in response to ER stress. SH-SY5Y cells were transfected with parkin or control siRNA duplexes. On day 2 after transfection, the cells were shifted to low-glucose medium containing 3 mM glucose instead of 25 mM. On day 3, the cells were treated with 2 _μ_g/ml tunicamycin (TM) for 5 h and the steady-state cellular ATP levels were measured by a bioluminescence assay. Cultured cells derived from tumors derive almost all of their energy from aerobic glycolysis rather than mitochondrial oxidative phosphorylation; in addition, stimulation of glycolysis in the presence of glucose inhibits mitochondrial respiration. Therefore, low glucose concentrations in the medium forces the cells to relay on oxidative phosphorylation to generate sufficient ATP. ***P<0.001, **P<0.01
Figure 9
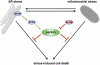
Interplay between ER stress, mitochondrial stress and parkin. ER stress can induce mitochondrial damage, such as alterations in mitochondrial morphology and bioenergetics. Conversely, mitochondrial stress can induce ER stress, reflected by the induction of the unfolded protein response (UPR). Parkin is transcriptionally upregulated in response to both mitochondrial and ER stress by ATF4, a transcription factor of the UPR. The stress-induced transcriptional upregulation of parkin is antagonized by c-Jun, which is activated by the JNK pathway. Increased expression of parkin under stress conditions protects cells from stress-induced cell death, explaining the high vulnerability of parkin-deficient cells to cellular stress
Similar articles
- Cell type-specific upregulation of Parkin in response to ER stress.
Wang HQ, Imai Y, Kataoka A, Takahashi R. Wang HQ, et al. Antioxid Redox Signal. 2007 May;9(5):533-42. doi: 10.1089/ars.2006.1522. Antioxid Redox Signal. 2007. PMID: 17465879 - Glutamate excitotoxicity in neurons triggers mitochondrial and endoplasmic reticulum accumulation of Parkin, and, in the presence of N-acetyl cysteine, mitophagy.
Van Laar VS, Roy N, Liu A, Rajprohat S, Arnold B, Dukes AA, Holbein CD, Berman SB. Van Laar VS, et al. Neurobiol Dis. 2015 Feb;74:180-93. doi: 10.1016/j.nbd.2014.11.015. Epub 2014 Dec 3. Neurobiol Dis. 2015. PMID: 25478815 Free PMC article. - Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability.
Méndez-Lucas A, Hyroššová P, Novellasdemunt L, Viñals F, Perales JC. Méndez-Lucas A, et al. J Biol Chem. 2014 Aug 8;289(32):22090-102. doi: 10.1074/jbc.M114.566927. Epub 2014 Jun 27. J Biol Chem. 2014. PMID: 24973213 Free PMC article. - [Etiology and pathogenesis of Parkinson's disease: from mitochondrial dysfunctions to familial Parkinson's disease].
Hattori N. Hattori N. Rinsho Shinkeigaku. 2004 Apr-May;44(4-5):241-62. Rinsho Shinkeigaku. 2004. PMID: 15287506 Review. Japanese. - The Role of the PERK/eIF2α/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. Rozpedek W, et al. Curr Mol Med. 2016;16(6):533-44. doi: 10.2174/1566524016666160523143937. Curr Mol Med. 2016. PMID: 27211800 Free PMC article. Review.
Cited by
- Parkin, an E3 Ubiquitin Ligase, Plays an Essential Role in Mitochondrial Quality Control in Parkinson's Disease.
Wang XL, Feng ST, Wang ZZ, Yuan YH, Chen NH, Zhang Y. Wang XL, et al. Cell Mol Neurobiol. 2021 Oct;41(7):1395-1411. doi: 10.1007/s10571-020-00914-2. Epub 2020 Jul 4. Cell Mol Neurobiol. 2021. PMID: 32623547 Review. - FosB and ΔFosB expression in brain regions containing differentially susceptible dopamine neurons following acute neurotoxicant exposure.
Patterson JR, Kim EJ, Goudreau JL, Lookingland KJ. Patterson JR, et al. Brain Res. 2016 Oct 15;1649(Pt A):53-66. doi: 10.1016/j.brainres.2016.08.030. Epub 2016 Aug 24. Brain Res. 2016. PMID: 27566062 Free PMC article. - Impaired complex IV activity in response to loss of LRPPRC function can be compensated by mitochondrial hyperfusion.
Rolland SG, Motori E, Memar N, Hench J, Frank S, Winklhofer KF, Conradt B. Rolland SG, et al. Proc Natl Acad Sci U S A. 2013 Aug 6;110(32):E2967-76. doi: 10.1073/pnas.1303872110. Epub 2013 Jul 22. Proc Natl Acad Sci U S A. 2013. PMID: 23878239 Free PMC article. - CD38 in SLE CD4 T cells promotes Ca2+ flux and suppresses interleukin-2 production by enhancing the expression of GM2 on the surface membrane.
Katsuyama E, Humbel M, Suarez-Fueyo A, Satyam A, Yoshida N, Kyttaris VC, Tsokos MG, Tsokos GC. Katsuyama E, et al. Nat Commun. 2024 Sep 27;15(1):8304. doi: 10.1038/s41467-024-52617-7. Nat Commun. 2024. PMID: 39333474 Free PMC article. - Editorial: Mitochondria and Endoplasmic Reticulum Dysfunction in Parkinson's Disease.
Barodia SK, Prabhakaran K, Karunakaran S, Mishra V, Tapias V. Barodia SK, et al. Front Neurosci. 2019 Nov 8;13:1171. doi: 10.3389/fnins.2019.01171. eCollection 2019. Front Neurosci. 2019. PMID: 31780882 Free PMC article. No abstract available.
References
- Winklhofer KF, Haass C. Mitochondrial dysfunction in Parkinson′s disease. Biochimica Et Biophysica Acta. 2010;1802:29–44. - PubMed
- Schapira AH. Mitochondria in the aetiology and pathogenesis of Parkinson's disease. Lancet Neurol. 2008;7:97–109. - PubMed
- Wang HQ, Takahashi R. Expanding insights on the involvement of endoplasmic reticulum stress in Parkinson's disease. Antioxid Redox Signal. 2007;9:553–561. - PubMed
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. - PubMed
- Ghribi O, Herman MM, Pramoonjago P, Savory J. MPP+ induces the endoplasmic reticulum stress response in rabbit brain involving activation of the ATF-6 and NF-kappaB signaling pathways. J Neuropathol Exp Neurol. 2003;62:1144–1153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous