Targeted insertion of cysteine by decoding UGA codons with mammalian selenocysteine machinery - PubMed (original) (raw)
Targeted insertion of cysteine by decoding UGA codons with mammalian selenocysteine machinery
Xue-Ming Xu et al. Proc Natl Acad Sci U S A. 2010.
Abstract
Cysteine (Cys) is inserted into proteins in response to UGC and UGU codons. Herein, we show that supplementation of mammalian cells with thiophosphate led to targeted insertion of Cys at the UGA codon of thioredoxin reductase 1 (TR1). This Cys was synthesized by selenocysteine (Sec) synthase on tRNA([Ser]Sec) and its insertion was dependent on the Sec insertion sequence element in the 3'UTR of TR1 mRNA. The substrate for this reaction, thiophosphate, was synthesized by selenophosphate synthetase 2 from ATP and sulfide and reacted with phosphoseryl-tRNA([Ser]Sec) to generate Cys-tRNA([Ser]Sec). Cys was inserted in vivo at UGA codons in natural mammalian TRs, and this process was regulated by dietary selenium and availability of thiophosphate. Cys occurred at 10% of the Sec levels in liver TR1 of mice maintained on a diet with normal amounts of selenium and at 50% in liver TR1 of mice maintained on a selenium deficient diet. These data reveal a novel Sec machinery-based mechanism for biosynthesis and insertion of Cys into protein at UGA codons and suggest new biological functions for thiophosphate and sulfide in mammals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
In vitro Cys synthesis on tRNA[Ser]Sec by SecS. All reactions were carried out under anaerobic conditions in the presence of mSecS (unless otherwise indicated) and _O_-phospho-3H-seryl-tRNA[Ser]Sec. Cys synthesis was monitored by adding (A) SPO3, (B) mSPS2, Na2S, and ATP, (C) mSPS2 and Na2S, (D) cSPS2, Na2S, and ATP, (E) SelD, Na2S, and ATP, or (F) a control protein, Trx, that included mSPS2, Na2S, and ATP showed no activity in synthesizing Cys-tRNA[Ser]Sec. Migration of amino acid standards is shown below each panel. Experimental details are given in Materials and Methods.
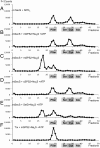
Fig. 2.
Sulfide-dependent ATP hydrolysis by selenophosphate synthetases (SPSs). ATP hydrolysis reactions were carried out using [α-32P]ATP in the presence or absence (designated NC, negative control) of either 5.0 mM sodium sulfide or 0.1 mM selenide. Three SPSs, mSPS2, cSPS2, and SelD, were examined. At the end of the incubation period, compounds were separated on PEI TLC plates and visualized by exposing to a PhosphorImager screen. Experimental details are given in Materials and Methods.
Fig. 3.
Cys insertion into TR1 in NIH 3T3 cells in the presence of SPO3. (A) NIH 3T3 cells were cultured without (lanes 1, 2, duplicates) or with (lanes 3, 4, duplicates) 1 mM SPO3, labeled with 75Se, and analyzed by SDS-PAGE for selenoprotein expression. (Upper) 75Se labeling is shown, (Center) Western blot analyses of TR1, GPx4, and GPx1 are shown, and (Lower) Coomassie blue staining of proteins, used as a loading control, is shown. Experimental details are given in Materials and Methods. (B) NIH 3T3 cells were transfected with pGFP (the control vector encoding pGFP), pGFP-TR1-His-SECIS (encoding pGFP, TR1 with a His-tag at the C terminus and the SECIS element), or pGFP-TR1-His (encoding pGFP, TR1 with a His-tag at the C terminus but minus a SECIS element), grown either without (lanes 1–3) or with (lanes 4–6) 1 mM SPO3, labeled with 75Se, and analyzed by SDS-PAGE. (Upper) 75Se labeling is shown, (Center) Western blot analysis with either anti-TR1 or anti-His antibody as designated is shown, and (Lower) Coomassie blue staining of proteins, used as a loading control, is shown. Experimental details are given in Materials and Methods.
Similar articles
- Biosynthesis of selenocysteine, the 21st amino acid in the genetic code, and a novel pathway for cysteine biosynthesis.
Turanov AA, Xu XM, Carlson BA, Yoo MH, Gladyshev VN, Hatfield DL. Turanov AA, et al. Adv Nutr. 2011 Mar;2(2):122-8. doi: 10.3945/an.110.000265. Epub 2011 Mar 10. Adv Nutr. 2011. PMID: 22332041 Free PMC article. Review. - Biosynthesis of selenocysteine on its tRNA in eukaryotes.
Xu XM, Carlson BA, Mix H, Zhang Y, Saira K, Glass RS, Berry MJ, Gladyshev VN, Hatfield DL. Xu XM, et al. PLoS Biol. 2007 Jan;5(1):e4. doi: 10.1371/journal.pbio.0050004. PLoS Biol. 2007. PMID: 17194211 Free PMC article. - UGA codon position affects the efficiency of selenocysteine incorporation into glutathione peroxidase-1.
Wen W, Weiss SL, Sunde RA. Wen W, et al. J Biol Chem. 1998 Oct 23;273(43):28533-41. doi: 10.1074/jbc.273.43.28533. J Biol Chem. 1998. PMID: 9774484 - Why is mammalian thioredoxin reductase 1 so dependent upon the use of selenium?
Lothrop AP, Snider GW, Ruggles EL, Hondal RJ. Lothrop AP, et al. Biochemistry. 2014 Jan 28;53(3):554-65. doi: 10.1021/bi400651x. Epub 2014 Jan 15. Biochemistry. 2014. PMID: 24393022 Free PMC article. - [Biochemical selenocysteine synthesis and the phylogenic study].
Mizutani T, Osaka T, Fujiwara T, Shahidzzman M. Mizutani T, et al. Yakugaku Zasshi. 2008 Jul;128(7):989-96. doi: 10.1248/yakushi.128.989. Yakugaku Zasshi. 2008. PMID: 18591866 Review. Japanese.
Cited by
- RadAA: A Command-line Tool for Identification of Radical Amino Acid Changes in Multiple Sequence Alignments.
Seim I, Baker AM, Chopin LK. Seim I, et al. Mol Inform. 2019 Jan;38(1-2):e1800057. doi: 10.1002/minf.201800057. Epub 2018 Jul 17. Mol Inform. 2019. PMID: 30019526 Free PMC article. - The pathological role of ferroptosis in ischemia/reperfusion-related injury.
Yan HF, Tuo QZ, Yin QZ, Lei P. Yan HF, et al. Zool Res. 2020 May 18;41(3):220-230. doi: 10.24272/j.issn.2095-8137.2020.042. Zool Res. 2020. PMID: 32314558 Free PMC article. Review. - Selenium and selenocysteine: roles in cancer, health, and development.
Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Hatfield DL, et al. Trends Biochem Sci. 2014 Mar;39(3):112-20. doi: 10.1016/j.tibs.2013.12.007. Epub 2014 Jan 28. Trends Biochem Sci. 2014. PMID: 24485058 Free PMC article. Review. - Recoding of the selenocysteine UGA codon by cysteine in the presence of a non-canonical tRNACys and elongation factor SelB.
Vargas-Rodriguez O, Englert M, Merkuryev A, Mukai T, Söll D. Vargas-Rodriguez O, et al. RNA Biol. 2018;15(4-5):471-479. doi: 10.1080/15476286.2018.1474074. Epub 2018 Jun 18. RNA Biol. 2018. PMID: 29879865 Free PMC article. - Ferroptosis: mechanisms and links with diseases.
Yan HF, Zou T, Tuo QZ, Xu S, Li H, Belaidi AA, Lei P. Yan HF, et al. Signal Transduct Target Ther. 2021 Feb 3;6(1):49. doi: 10.1038/s41392-020-00428-9. Signal Transduct Target Ther. 2021. PMID: 33536413 Free PMC article. Review.
References
- Arner ES. Selenoproteins—What unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res. 2010;316:1296–1303. - PubMed
- Stadtman TC. Selenocysteine. Annu Rev Biochem. 1996;65:83–100. - PubMed
- Lu J, Holmgren A. Selenoproteins. J Biol Chem. 2009;284:723–727. - PubMed
- Low SC, Berry MJ. Knowing when not to stop: Selenocysteine incorporation in eukaryotes. Trends Biochem Sci. 1996;21:203–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 GM065204/GM/NIGMS NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- GM065204/GM/NIGMS NIH HHS/United States
- R01 GM065204/GM/NIGMS NIH HHS/United States
- R01 GM061603/GM/NIGMS NIH HHS/United States
- GM061603/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources