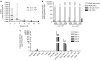Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration - PubMed (original) (raw)
doi: 10.1038/mt.2010.258. Epub 2010 Nov 30.
Michael Lukason, Margaret Collins, Robert Munger, Elisabete Isenberger, Cindy Rogers, Shana Malatos, Elizabeth Dufresne, James Morris, Roberto Calcedo, Gabor Veres, Abraham Scaria, Laura Andrews, Samuel Wadsworth
Affiliations
- PMID: 21119620
- PMCID: PMC3034852
- DOI: 10.1038/mt.2010.258
Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration
Timothy K Maclachlan et al. Mol Ther. 2011 Feb.
Abstract
AAV2-sFLT01 is a vector that expresses a modified soluble Flt1 receptor designed to neutralize the proangiogenic activities of vascular endothelial growth factor (VEGF) for treatment of age-related macular degeneration (AMD) via an intravitreal injection. Owing to minimal data available for the intravitreal route of administration for adeno-associated virus (AAV), we initiated a 12-month safety study of AAV2-sFLT01 administered intravitreally at doses of 2.4 × 10(9) vector genomes (vg) and 2.4 × 10(10) vg to cynomolgus monkeys. Expression of sFlt01 protein peaked at ~1-month postadministration and remained relatively constant for the remainder of the study. Electroretinograms, fluorescein angiograms, and tonometry were assessed every 3 months, with no test article-related findings observed in any group. Indirect ophthalmoscopy and slit lamp exams performed monthly revealed a mild to moderate but self-resolving vitreal inflammation in the high-dose group only, which follow-up studies suggest was directed against the AAV2 capsid. Histological evaluation revealed no structural changes in any part of the eye and occasional inflammatory cells in the trabecular meshwork, vitreous and retina in the high-dose group. Biodistribution analysis in rats and monkeys found only trace amounts of vector outside the injected eye. In summary, these studies found AAV2-sFLT01 to be well-tolerated, localized, and capable of long-term expression.
Figures
Figure 1
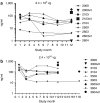
Expression of sFlt01 protein in the aqueous humor of the eye. Aqueous humor was sampled from the injected (right) eye of nonhuman primates at 1, 3, 6, 9, and 12 months postinjection of AAV2-sFLT01 from (a) group 2 (2.4 × 109 vg) or from (b) group 3 (2.4 × 1010 vg). One animal in group 3 (#3603) was kept on study for an additional 6 months. At the end of the study, the eyes from animals 2009, 2103, 2603, 3009, 3603, and 3604 were dissected for vitreous collection (“vit”) and were assayed for sFlt01 expression. AAV2, adeno-associated virus type 2; vg, vector genomes.
Figure 2
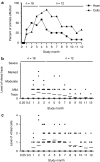
Vitreal inflammation observed in 2 × 1010 vg dosing group. Animals on study were examined by indirect ophthalmoscopy. 2 × 1010 vg dosed animals were found to develop inflammation over the course of the study, with onset in the majority of animals by 3 months (a). Severity averaged between trace and mild haze (b) and a grade 1–2 cells (c) with some animals displaying marked haze and/or higher grades of cells at brief points during the study. vg, vector genomes.
Figure 3
Inflammatory cells in the eye. At both the 3- and 12-month sacrifice, inflammatory cells were seen in the eye, most often commensurate with ophthalmologic exams performed on the same animal. At the 3-month sacrifice, lymphocytes were seen in the (a) trabecular meshwork. At the 12-month sacrifice, lymphocytes and macrophages were observed within and adjacent to the pars plana of the (b) ciliary body and pigmented cells within the (c) trabecular meshwork. Retinal integrity was also evaluated in (d) vehicle and (e,f) high-dose animals. At no point was any damage noted to ocular structures.
Figure 4
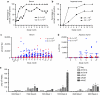
Source of intravitreal inflammation. Fifteen nonhuman primates were injected with 2.4 × 1010 vg of AAV2-sFLT01 into the right eye. Five animals were dosed with AAV2 vector with no transgene (AAV2-null), five dosed with AAV2-sFLT01 prepared by the HSV-based production system, identical to the preparation used in the 12-month toxicology study (AAV2-sFLT01-HSV) and five were dosed with a preparation of AAV2-sFLT01 made by transfection methods lacking any HSV component (AAV2-sFLT01-tfxn). Varying degrees of inflammation as noted by (a) vitreal haze and cells were observed in all groups. AAV2, adeno-associated virus type 2; HSV, herpes simplex virus; vg, vector genomes.
Figure 5
Immunogenicity against AAV2 capsid protein. Serum and aqueous humor were sampled at various intervals throughout the course of the 12-month toxicology study and analyzed for total IgG titer against AAV2 capsid. There was a dose- and time-dependent response and the majority of animals that would go on to develop anti-AAV2 antibodies seroconverted in both the serum and aqueous by (a,b) 3 months postinjection. Varying levels of anti-AAV2 antibodies were observed, with higher levels seen in the (c) serum compared to the (d) aqueous. In the source of inflammation study, T-cell responses (indicated by IFN-γ production) against AAV2 and sFlt01 were examined at various timepoints (e). Here, results from week-1 and week 8 postinjection samples, where responses were maximal, are shown. Pools of peptides representing overlapping sequence of sFlt01 (sFlt01A, sFlt01B) and AAV2 capsid protein (AAV2A, AAV2B, AAV2C) were incubated with PBMCs and were analyzed for intracellular expression of interferon-γ by ELISpot analysis. Bars marked with an *indicate a significant induction wells where more the amount of γ-interferon was greater than three times that measured in the “negative control” cells and the value, in SFU/106 PBMCs, was >55. Animals 1502 and 1503 were in group 1 in this study, and received 2.4 × 1010 vg/eye of HSV-method produced AAV2-sFLT01. Animal 3503 was in group 3 in this study, and received 2.4 × 1010 vg/eye of transfection-method produced AAV2-sFLT01. AAV2, adeno-associated virus type 2; ELISpot, enzyme-linked immunosorbent spot; HSV, herpes simplex virus; IgG, immunoglobulin G; IFN, interferon; PBMC, peripheral blood mononuclear cell; SFU, spot-forming units; vg, vector genomes.
Figure 6
Biodistribution of AAV2-sFLT01. (a) Animals from the 12-month toxicology study were sampled for serum every 3 months and processed for total DNA. Vector-specific AAV2-sFLT01 sequences were amplified by PCR. The total copy number of AAV2-sFLT01 was normalized to copy number of the endogenous gene CFTR. (b) At the terminal sacrifice, the retina and optic nerve of three animals each from groups 2 and 3 of the “Ocular Sampling” group were harvested, processed for DNA, and samples were amplified by PCR for unique AAV2-sFLT01 sequences. (c) An additional study was performed in rat to more comprehensively evaluate the spread of AAV2-sFLT01. In this one hundred animal study rats were dosed with 6.4 × 107 vg AAV2-sFLT01 or vehicle into the right eye. Ten animals from each group were sacrificed and necropsied for various organs and blood at 3, 8, 15, 31, and 92 days postinjection. Total DNA was prepared from each tissue and unique AAV2-sFLT01 sequences were amplified by PCR. Copies detected of AAV2-sFLT01 are reported as copies per µg genomic DNA. AAV2, adeno-associated virus type 2.
Similar articles
- Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: a phase 1, open-label trial.
Heier JS, Kherani S, Desai S, Dugel P, Kaushal S, Cheng SH, Delacono C, Purvis A, Richards S, Le-Halpere A, Connelly J, Wadsworth SC, Varona R, Buggage R, Scaria A, Campochiaro PA. Heier JS, et al. Lancet. 2017 Jul 1;390(10089):50-61. doi: 10.1016/S0140-6736(17)30979-0. Epub 2017 May 17. Lancet. 2017. PMID: 28526489 Clinical Trial. - Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule.
Lukason M, DuFresne E, Rubin H, Pechan P, Li Q, Kim I, Kiss S, Flaxel C, Collins M, Miller J, Hauswirth W, Maclachlan T, Wadsworth S, Scaria A. Lukason M, et al. Mol Ther. 2011 Feb;19(2):260-5. doi: 10.1038/mt.2010.230. Epub 2010 Oct 26. Mol Ther. 2011. PMID: 20978476 Free PMC article. - Development of gene therapy for treatment of age-related macular degeneration.
Askou AL. Askou AL. Acta Ophthalmol. 2014 Jul;92 Thesis3:1-38. doi: 10.1111/aos.12452. Acta Ophthalmol. 2014. PMID: 24953666 - Gene therapy for age-related macular degeneration.
Moore NA, Bracha P, Hussain RM, Morral N, Ciulla TA. Moore NA, et al. Expert Opin Biol Ther. 2017 Oct;17(10):1235-1244. doi: 10.1080/14712598.2017.1356817. Epub 2017 Jul 20. Expert Opin Biol Ther. 2017. PMID: 28726562 Review. - Gene Therapy for Non-Hereditary Retinal Disease: Age-Related Macular Degeneration, Diabetic Retinopathy, and Beyond.
Rowe LW, Ciulla TA. Rowe LW, et al. Genes (Basel). 2024 Jun 1;15(6):720. doi: 10.3390/genes15060720. Genes (Basel). 2024. PMID: 38927656 Free PMC article. Review.
Cited by
- Proteasome inhibition is partially effective in attenuating pre-existing immunity against recombinant adeno-associated viral vectors.
Karman J, Gumlaw NK, Zhang J, Jiang JL, Cheng SH, Zhu Y. Karman J, et al. PLoS One. 2012;7(4):e34684. doi: 10.1371/journal.pone.0034684. Epub 2012 Apr 13. PLoS One. 2012. PMID: 22514654 Free PMC article. - Gene Therapy Intervention in Neovascular Eye Disease: A Recent Update.
Lin FL, Wang PY, Chuang YF, Wang JH, Wong VHY, Bui BV, Liu GS. Lin FL, et al. Mol Ther. 2020 Oct 7;28(10):2120-2138. doi: 10.1016/j.ymthe.2020.06.029. Epub 2020 Jun 30. Mol Ther. 2020. PMID: 32649860 Free PMC article. Review. - A comprehensive review of retinal gene therapy.
Boye SE, Boye SL, Lewin AS, Hauswirth WW. Boye SE, et al. Mol Ther. 2013 Mar;21(3):509-19. doi: 10.1038/mt.2012.280. Epub 2013 Jan 29. Mol Ther. 2013. PMID: 23358189 Free PMC article. Review. - Advances in Gene Therapy for Diseases of the Eye.
Petit L, Khanna H, Punzo C. Petit L, et al. Hum Gene Ther. 2016 Aug;27(8):563-79. doi: 10.1089/hum.2016.040. Epub 2016 Jun 13. Hum Gene Ther. 2016. PMID: 27178388 Free PMC article. Review. - Transduction Patterns of Adeno-associated Viral Vectors in a Laser-Induced Choroidal Neovascularization Mouse Model.
Lee SH, Kim YS, Nah SK, Kim HJ, Park HY, Yang JY, Park K, Park TK. Lee SH, et al. Mol Ther Methods Clin Dev. 2018 Jan 31;9:90-98. doi: 10.1016/j.omtm.2018.01.008. eCollection 2018 Jun 15. Mol Ther Methods Clin Dev. 2018. PMID: 29766021 Free PMC article.
References
- Gottlieb JL. Age-related macular degeneration. JAMA. 2002;288:2233–2236. - PubMed
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. - PubMed
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, ANCHOR Study Group et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, MARINA Study Group et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical