Extracellular matrix: functions in the nervous system - PubMed (original) (raw)
Review
Extracellular matrix: functions in the nervous system
Claudia S Barros et al. Cold Spring Harb Perspect Biol. 2011.
Abstract
An astonishing number of extracellular matrix glycoproteins are expressed in dynamic patterns in the developing and adult nervous system. Neural stem cells, neurons, and glia express receptors that mediate interactions with specific extracellular matrix molecules. Functional studies in vitro and genetic studies in mice have provided evidence that the extracellular matrix affects virtually all aspects of nervous system development and function. Here we will summarize recent findings that have shed light on the specific functions of defined extracellular matrix molecules on such diverse processes as neural stem cell differentiation, neuronal migration, the formation of axonal tracts, and the maturation and function of synapses in the peripheral and central nervous system.
Figures
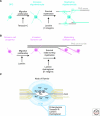
Figure 1.
ECM molecules in the developing neocortex. (A) Overview of some ECM molecules found in the embryonic neocortex. Laminin (LN) is a major component of the basal lamina (BL) under the pia mater (P) and is also found in the ventricular zone (VZ). Reelin (RLN) is secreted in the marginal zone (MZ) by Cajal-Retzius cells. Chondroitin sulfate proteoglycans (CSPGs) are concentrated in the subplate region above the intermediate zone (IZ). (B) Higher magnification schematic of the boxed region in (A). RGC endfeet interact with ECM molecules in the BL, such as LN and perlecan (PN), through the integrin (ITG) and dystroglycan (DG) receptors. Radial glia and neurons engage in reelin signaling via the ApoER2 (AP) and VLDLR (VL) receptors.
Figure 2.
ECM and myelination. (A) Oligodendroglia differentiate in sequential stages to generate mature oligodendrocytes. Each oligodendrocyte myelinates several CNS axons. Tenascin-C, laminin, and their β1 integrin receptors play roles at different developmental stages, as indicated. (B) Schwann cells myelinate peripheral nerves. Immature Schwann cells sort out axonal bundles to individually myelinate each axon. Laminin regulates all stages of Schwann cell development, whereas dystroglycan and β1 integrin receptors control axonal sorting and myelination. (C) The ECM surrounding nodes of Ranvier may regulate the local concentration of cations and clusters voltage-gated sodium channels, which allow for saltatory electrical conductivity. Several proteoglycans, tenascin-R, laminin and dystroglycan contribute to the formation of nodal matrices. Nav, voltage-gated channel; Na+, sodium cations.
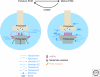
Figure 3.
ECM molecules at the neuromuscular junction. ECM molecules (BL) are required for NMJ development and function. The heparan sulfate proteoglycan agrin binds to its receptor, Lrp4, and regulates postsynaptic NMJ development through the receptor tyrosine kinase, MuSK. Laminins (LN) are required at the NMJ to promote presynaptic differentiation, as well as postsynaptic maturation via integrin (ITG) and α-dystroglycan (DG) receptors. ITG and DG receptors also bind perlecan (PN) in the BL, which recruits collagen Q (ColQ). ColQ can also bind MuSK and is important for AchR clustering and regulation of Ach levels via recruitment of acetylcholinesterase (AchE) to the NMJ.
Figure 4.
ECM changes at CNS synapses. Synapses are embedded into an ECM meshwork (blue) composed of hyaluronan, chondroitin sulfate proteglycans (CSPGs), tenascins, and others. The composition of the ECM changes during development. For example, neurocan, versican V1, and tenascin-C are abundant in the immature CNS, whereas tenascin-R, versican V2, and Bral1 are prominent in the mature CNS. The mature ECM is thought to restrict dendritic spine motility and lateral diffusion of AMPA receptors (AMPAr). Chondroitinase ABC (chABC) digestion of CSPGs can restore juvenile spine dynamics.
Similar articles
- Roles of Reelin/Disabled1 pathway on functional recovery of hemiplegic mice after neural cell transplantation; Reelin promotes migration toward motor cortex and maturation to motoneurons of neural grafts.
Arimitsu N, Takai K, Fujiwara N, Shimizu J, Ueda Y, Wakisaka S, Hirotsu C, Murayama MA, Suzuki T, Suzuki N. Arimitsu N, et al. Exp Neurol. 2019 Oct;320:112970. doi: 10.1016/j.expneurol.2019.112970. Epub 2019 Jun 8. Exp Neurol. 2019. PMID: 31185198 - Components of astrocytic extracellular matrix are regulated by contact with axons.
Ard MD, Faissner A. Ard MD, et al. Ann N Y Acad Sci. 1991;633:566-9. doi: 10.1111/j.1749-6632.1991.tb15663.x. Ann N Y Acad Sci. 1991. PMID: 1724133 No abstract available. - The extracellular matrix provides directional cues for neuronal migration during cerebellar development.
Porcionatto MA. Porcionatto MA. Braz J Med Biol Res. 2006 Mar;39(3):313-20. doi: 10.1590/s0100-879x2006000300001. Epub 2006 Feb 22. Braz J Med Biol Res. 2006. PMID: 16501810 Review. - Cytotactin and cytotactin-binding proteoglycan. An interactive pair of extracellular matrix proteins.
Hoffman S, Crossin KL, Jones FS, Friedlander DR, Edelman GM. Hoffman S, et al. Ann N Y Acad Sci. 1990;580:288-301. doi: 10.1111/j.1749-6632.1990.tb17938.x. Ann N Y Acad Sci. 1990. PMID: 1692457 Review. No abstract available. - [Functions of Reelin in cortical neuron migration].
Kohno T, Hattori M. Kohno T, et al. Seikagaku. 2016 Feb;88(1):105-13. Seikagaku. 2016. PMID: 27025013 Review. Japanese. No abstract available.
Cited by
- Dissection of the Genetic Association between Anorexia Nervosa and Obsessive-Compulsive Disorder at the Network and Cellular Levels.
Song W, Wang W, Yu S, Lin GN. Song W, et al. Genes (Basel). 2021 Mar 27;12(4):491. doi: 10.3390/genes12040491. Genes (Basel). 2021. PMID: 33801746 Free PMC article. - Generation of retinal ganglion cells with functional axons from human induced pluripotent stem cells.
Tanaka T, Yokoi T, Tamalu F, Watanabe S, Nishina S, Azuma N. Tanaka T, et al. Sci Rep. 2015 Feb 10;5:8344. doi: 10.1038/srep08344. Sci Rep. 2015. PMID: 25666360 Free PMC article. - A patient-specific induced pluripotent stem cell model for West syndrome caused by ST3GAL3 deficiency.
van Diepen L, Buettner FFR, Hoffmann D, Thiesler CT, von Bohlen Und Halbach O, von Bohlen Und Halbach V, Jensen LR, Steinemann D, Edvardson S, Elpeleg O, Schambach A, Gerardy-Schahn R, Kuss AW. van Diepen L, et al. Eur J Hum Genet. 2018 Dec;26(12):1773-1783. doi: 10.1038/s41431-018-0220-5. Epub 2018 Aug 8. Eur J Hum Genet. 2018. PMID: 30089820 Free PMC article. - Engineering geometrical 3-dimensional untethered in vitro neural tissue mimic.
Pagan-Diaz GJ, Ramos-Cruz KP, Sam R, Kandel ME, Aydin O, Saif MTA, Popescu G, Bashir R. Pagan-Diaz GJ, et al. Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25932-25940. doi: 10.1073/pnas.1916138116. Epub 2019 Dec 3. Proc Natl Acad Sci U S A. 2019. PMID: 31796592 Free PMC article. - The role of neural stem cells in regulating glial scar formation and repair.
Nicaise AM, D'Angelo A, Ionescu RB, Krzak G, Willis CM, Pluchino S. Nicaise AM, et al. Cell Tissue Res. 2022 Mar;387(3):399-414. doi: 10.1007/s00441-021-03554-0. Epub 2021 Nov 25. Cell Tissue Res. 2022. PMID: 34820704 Free PMC article. Review.
References
- Adams JC, Tucker RP 2000. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn 218: 280–299 - PubMed
- Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y 2002. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci 5: 119–123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 MH078833/MH/NIMH NIH HHS/United States
- MH078833/MH/NIMH NIH HHS/United States
- F32 NS060355/NS/NINDS NIH HHS/United States
- R01 NS046456/NS/NINDS NIH HHS/United States
- NS060355/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources