Leukocytes in mammary development and cancer - PubMed (original) (raw)
Review
Leukocytes in mammary development and cancer
Lisa M Coussens et al. Cold Spring Harb Perspect Biol. 2011.
Abstract
Leukocytes, of both the innate and adaptive lineages, are normal cellular components of all tissues. These important cells not only are critical for regulating normal tissue homeostasis, but also are significant paracrine regulators of all physiologic and pathologic tissue repair processes. This article summarizes recent insights regarding the trophic roles of leukocytes at each stage of mammary gland development and during cancer development, with a focus on Murids and humans.
Figures
Figure 1.
Macrophage and eosinophil recruitment to the terminal end buds of mice. H&E longitudinal sections of terminal end buds at 5 wk of age. Sections were first stained with H&E (A–C) and then destained and immunostained using anti-F4/80 antibody followed by a peroxidase detection system for positive signal (brown; D,E,F). Note the presence of a dense stroma particularly around the shaft and beginning of the TEB head that consists of fibroblasts and abundant immune cells. This stroma isolates the epithelial compartment from the adipoctyes of the fat pad but is sparser at the growing tip. B,C, and E,F are high-powered views of A and D, respectively, and the lower panels boxed in A and D are shown in B and E whereas the upper panels are in C and F. The immunostain indicates the F4/80 positive macrophages (arrows) and eosinophils (filled arrow heads) the latter recognized by their eosinophilic granules and polymorphonuclear structures in C. Note the distinct but overlapping localization of macrophages and eosinophils around the bulbous head and shaft of the TEB. (Figure adapted from Gouon-Evans et al. [2000] and reprinted here with permission from The Company of Biologists © 2000.)
Figure 2.
Distribution of mast cells around the terminal end bud of mice. Shown are formalin fixed, paraffin embedded sections stained with toluidine blue in which mast cells are identified by an enzymatic reaction to detect chymase a defining enzyme of these cells. (A) Mast cells (red arrows) shown in the fatty stroma in front of an invading terminal end bud (TEB]. (B) Many mast cells (red arrows) adjacent to developing mammary epithelium of mice at 5 wk of age. Black arrow shows a mast cell degranulating. (Panel A reprinted from Lilla and Werb [2010] and reprinted here with permission from Elsevier © 2010; panel B kindly provided by Dr. Zena Werb, USCF). Bar 50 µm.
Figure 3.
Association of macrophages and collagen fibers with the terminal end bud. Multiphoton imaging of frozen sections of terminal end buds with nuclei stained with DAPI in which the collagen fibers are shown by second harmonic residence and pseudo-colored in green while macrophages are shown by expression of GFP from the _Cs1r_-promoter (data from Sasmono et al. 2003) pseudocolored to red. In A and B, TEBs from mice heterozygous (+/−) for the _Csf1_op allele, whereas C is from mice homozygous (−/−) for this allele. Note the sheafing of the TEB with collagen 1 containing fibers and the association of macrophages with these fibers. The tubular structure with the visible collagen sheaf running laterally across the image in B is a blood vessel. In C, the collagen fibers are more disorganized and the TEB is rounder in structure (data from Ingman et al. 2006).
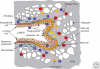
Figure 4.
Topography of immune cells in the developing terminal end bud. The diagram shows a schematic of a terminal end bud (TEB) that is bifurcating to give two ductal branches. This TEB is surrounded by a dense fibroblastic stoma and encased by a fibrillar collagen network that is aligned in the direction of the outgrowth through the mammary fat pad that is densely populated by adipocytes. Abundant numbers of innate immune cells are recruited to the TEB and have preferred domains as indicated. Macrophages are enriched around the base and shaft of the TEB but move rapidly up and down the collagen fibers. In addition, they are found in the TEB itself where they phagocytose the apoptotic epithelial cells in the process of lumen formation. Mast cells in contrast, are preferentially located in the stroma in front of the invading TEBs where they provide proteases that enhance TEB invasion. Eosinophils in turn, are found around the bulbous head of the TEB and also are found in the cleft of the bifurcating TEB. Genetic ablation of each of these cell types as described in the text indicates roles for them in the branching morphogenesis of the mammary gland and their combined functions results in a fully branched ducal tree that forms during puberty.
Figure 5.
Leukocytes in human breast, ductal carcinoma in situ, and invasive carcinoma. (A) Hematoxylin and Eosin (H&E; top row) staining of normal human breast tissue, ductal carcinoma in situ (DCIS) and invasive breast cancer (invasive), showing ductal epithelial structures (E), collagenous stroma (C), and darkly stained immune cells infiltrating stroma (S). Immunodetection of infiltrating leukocytes by CD45 (leukocyte common antigen) immunoreactivity (brown staining; bottom row) reveals significant leukocyte infiltration in DCIS and invasive cancer, as compared to normal breast tissue. (B) Imunodetection of specific lineages of immune cells in invasive breast cancer, B lymphocytes (CD20+; brown staining), CD4+ T lymphocytes (brown staining), CD8+ T lymphocytes (brown staining), macrophages (CD68+; brown staining), neutrophils (neutrophils elastase+; brown staining), and mast cells (chymase+; blue staining). Original magnifications are shown for each panel.
Comment in
- On leukocytes in mammary development and cancer.
Ghajar CM. Ghajar CM. Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a013276. doi: 10.1101/cshperspect.a013276. Cold Spring Harb Perspect Biol. 2012. PMID: 22550234 Free PMC article. Review.
Similar articles
- Immune Cell Contribution to Mammary Gland Development.
Vickers R, Porter W. Vickers R, et al. J Mammary Gland Biol Neoplasia. 2024 Aug 23;29(1):16. doi: 10.1007/s10911-024-09568-y. J Mammary Gland Biol Neoplasia. 2024. PMID: 39177859 Free PMC article. Review. - On leukocytes in mammary development and cancer.
Ghajar CM. Ghajar CM. Cold Spring Harb Perspect Biol. 2012 May 1;4(5):a013276. doi: 10.1101/cshperspect.a013276. Cold Spring Harb Perspect Biol. 2012. PMID: 22550234 Free PMC article. Review. - Immune cell location and function during post-natal mammary gland development.
Reed JR, Schwertfeger KL. Reed JR, et al. J Mammary Gland Biol Neoplasia. 2010 Sep;15(3):329-39. doi: 10.1007/s10911-010-9188-7. Epub 2010 Aug 24. J Mammary Gland Biol Neoplasia. 2010. PMID: 20730636 Free PMC article. Review. - Macrophages: Regulators of the Inflammatory Microenvironment during Mammary Gland Development and Breast Cancer.
Brady NJ, Chuntova P, Schwertfeger KL. Brady NJ, et al. Mediators Inflamm. 2016;2016:4549676. doi: 10.1155/2016/4549676. Epub 2016 Jan 17. Mediators Inflamm. 2016. PMID: 26884646 Free PMC article. Review. - T cells are the main population in mouse breast milk and express similar profiles of tight junction proteins as those in mammary alveolar epithelial cells.
Ikebuchi R, Fujimoto M, Moriya T, Kusumoto Y, Kobayashi K, Tomura M. Ikebuchi R, et al. J Reprod Immunol. 2020 Aug;140:103137. doi: 10.1016/j.jri.2020.103137. Epub 2020 Apr 21. J Reprod Immunol. 2020. PMID: 32402923
Cited by
- Microbial Biomarkers in Liquid Biopsy for Cancer: An Overview and Future Directions.
Luo F, Wang X, Ye C, Sun H. Luo F, et al. Cancer Control. 2024 Jan-Dec;31:10732748241292019. doi: 10.1177/10732748241292019. Cancer Control. 2024. PMID: 39431347 Free PMC article. Review. - Loss of STING impairs lactogenic differentiation.
Vickers RR, Wyatt GL, Sanchez L, VanPortfliet JJ, West AP, Porter WW. Vickers RR, et al. Development. 2024 Oct 1;151(19):dev202998. doi: 10.1242/dev.202998. Epub 2024 Oct 14. Development. 2024. PMID: 39399905 Free PMC article. - Treg Cell Therapeutic Strategies for Breast Cancer: Holistic to Local Aspects.
Zhang H, Felthaus O, Eigenberger A, Klein S, Prantl L. Zhang H, et al. Cells. 2024 Sep 11;13(18):1526. doi: 10.3390/cells13181526. Cells. 2024. PMID: 39329710 Free PMC article. Review. - Association between breastfeeding, mammographic density, and breast cancer risk: a review.
Ye DM, Bai X, Xu S, Qu N, Zhao N, Zheng Y, Yu T, Wu H. Ye DM, et al. Int Breastfeed J. 2024 Sep 16;19(1):65. doi: 10.1186/s13006-024-00672-7. Int Breastfeed J. 2024. PMID: 39285438 Free PMC article. Review. - Immune Cell Contribution to Mammary Gland Development.
Vickers R, Porter W. Vickers R, et al. J Mammary Gland Biol Neoplasia. 2024 Aug 23;29(1):16. doi: 10.1007/s10911-024-09568-y. J Mammary Gland Biol Neoplasia. 2024. PMID: 39177859 Free PMC article. Review.
References
- Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjanen K 1992. Tumor size, nuclear morphometry, mitotic indices as prognostic factors in axillary-lymph-node-positive breast cancer. Eur Surg Res 24: 160–168 - PubMed
- American Cancer Society 2007. Cancer facts and figures. http://www.cancer.org/downloads/STT/caff2007PWSecured.pdf
- Anbazhagan R, Bartek J, Monaghan P, Gusterson BA 1991. Growth and development of the human infant breast. Am J Anat 192: 407–417 - PubMed
- Arribas J, Lopez-Casillas F, Massague J 1997. Role of the juxtamembrane domains of the transforming growth factor-α precursor and the β-amyloid precursor protein in regulated ectodomain shedding. J Biol Chem 272: 17160–17165 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical