NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease - PubMed (original) (raw)
Comparative Study
NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease
Richa B Tripathi et al. J Neurosci. 2010.
Abstract
The adult mammalian brain and spinal cord contain glial precursors that express platelet-derived growth factor receptor α subunit (PDGFRA) and the NG2 proteoglycan. These "NG2 cells" descend from oligodendrocyte precursors in the perinatal CNS and continue to generate myelinating oligodendrocytes in the gray and white matter of the postnatal brain. It has been proposed that NG2 cells can also generate reactive astrocytes at sites of CNS injury or demyelination. To test this we examined the fates of PDGFRA/NG2 cells in the mouse spinal cord during experimental autoimmune encephalomyelitis (EAE)--a demyelinating condition that models some aspects of multiple sclerosis in humans. We administered tamoxifen to Pdgfra-CreER(T2):Rosa26R-YFP mice to induce yellow fluorescent protein (YFP) expression in PDGFRA/NG2 cells and their differentiated progeny. We subsequently induced EAE and observed a large (>4-fold) increase in the local density of YFP(+) cells, >90% of which were oligodendrocyte lineage cells. Many of these became CC1-positive, NG2-negative differentiated oligodendrocytes that expressed myelin markers CNP and Tmem10/Opalin. PDGFRA/NG2 cells generated very few GFAP(+)-reactive astrocytes (1-2% of all YFP(+) cells) or NeuN(+) neurons (<0.02%). Thus, PDGFRA/NG2 cells act predominantly as a reservoir of new oligodendrocytes in the demyelinated spinal cord.
Figures
Figure 1.
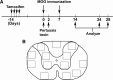
A, Timeline of the experiments. Mice were given tamoxifen (300 mg/kg) in corn oil by oral gavage on 4 consecutive days, starting 14 d before EAE induction. MOG peptide (amino acids 35–55) together with Freund's adjuvant was injected subcutaneously on days 0 and 7. Pertussis toxin (300 ng/ml) was injected intraperitoneally on days 0 and 2. Animals were analyzed on days 14 and 24 (males) or 28 (females). B, Diagram of the spinal cord indicating locations of sample boxes used for cell counts.
Figure 2.
Time course of EAE symptoms in male and female mice. A, Locomotor function of all mice deteriorated over the course of the study, with EAE-only control males (n = 7) responding on a similar course as control females (n = 10). B, A trend toward greater disability was apparent in Tam+EAE (n = 5) compared with EAE-only (n = 7) males, although the difference did not reach statistical significance. However, Tam+EAE males reached greater disability faster than EAE-only males and were humanely killed 24 d after EAE induction (24 dpi). C, In contrast, Tam+EAE females (n = 10) performed significantly better than EAE-only females (n = 10) (p < 0.0001). Also, the time of onset of symptoms was significantly delayed, by around a day, in Tam+EAE females. All data are represented as mean ± SEM. Two-way ANOVA was used to analyze data.
Figure 3.
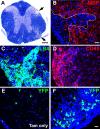
MOG immunization causes inflammation and demyelination. A–F, Following MOG immunization, demyelinated plaques, visible by local reductions of solochrome staining intensity (A, arrows) or loss of MBP immunoreactivity (dotted line in B) were seen at all spinal cord levels. Also, massive inflammation marked by CD45 (C) and ILB4 (D) labeling indicated activation of microglia and macrophages and infiltration by blood-borne immune cells. Following EAE induction there was an increase in YFP+ (_Pdgfra-_derived) cells (F) in Tam+EAE compared with Tam-only spinal cords (E). Images were taken from thoracic-lumbar spinal cord at 24 dpi in Tam+EAE animals unless indicated otherwise. Sections B–F are counterstained with Hoechst dye (blue). Scale bars: 200 μm (A), 20 μm (B, F).
Figure 4.
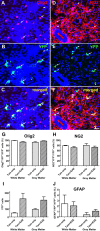
A large proportion of YFP+ cells belong to the OL lineage. Immunolabeling for OLIG2 (A) and YFP (B) revealed many colabeled cell (C), indicating they belonged to the OL lineage. This image was taken near a white matter lesion at 14 d dpi. Also, many YFP+ cells (E) coexpressed NG2 (D, F). The image was taken in the gray matter at 28 dpi. Arrows indicate double-labeled cells and arrowheads indicate single labeled cells. G, Quantification showed that 92–98% of all YFP+ cells were OLIG2+. There were no detectable differences between white or gray matter or between Tam+EAE and Tam-only groups (n = 5/group). H, NG2 cells comprised ∼78% of all YFP+ Tam-only cells and ∼72% in Tam+EAE white and gray matter (n = 3/group). I, As expected, there was a large increase in YFP+ cells in the white and gray matter following EAE induction. Data in I are shown as average numbers of YFP+ cells counted /animal. J, Approximately 2% and 1% of all YFP+ cells in the white and gray matter, respectively, were colabeled with GFAP (n = 5/group). All data are pooled from 14 d and 24 d/28 d post-EAE immunization (28 d and 38 d/42 d following tamoxifen administration) and represented as mean ± SEM. One-way ANOVA was used to compare data across groups. Scale bar, 20 μm.
Figure 5.
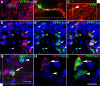
PDGFRA/NG2 cells give rise to mature OLs. A–C, A proportion of YFP+ cells colabels for CNP. The box in A is shown at higher magnification (single scan) in B along with an XZ plane through the presumptive myelin sheath. Another CNP+, YFP+ cell in the YZ and XZ planes is shown in C. Multiple CC1+, YFP+ oligodendrocytes are observed following EAE induction (D–F). These are shown at higher magnification in G–I. Orthogonal views are shown in G. Arrows indicate double-labeled cells and arrowheads single-labeled YFP+ cells. The CC1 images were taken from 24 dpi tissue and the CNP images from 28 dpi tissue. Sections are counterstained with Hoechst dye (Hst, blue). Scale bar, 20 μm.
Figure 6.
PDGFRA/NG2 glia give rise to myelinating cells. Processes from Opalin+, YFP+ cells in close apposition to Neurofilament (NF) profiles (A, B, arrows). Many Opalin+, YFP+ double-labeled cells were observed following EAE induction (C–E), some of which were found closely apposed to Neurofilament (NF) immunolabeling (A, B). Since Opalin is expressed in late-stage, myelinating OLs, this suggests that PDGFRA/NG2 cells give rise to remyelinating OLs in EAE. YFP+ cells that were colabeled for Ermin/Juxtanodin were also found in close association with NF+ profiles (F–H). MBP+ processes of YFP+ cells were sometimes found entwined with NF+ processes (I–K). Arrows indicate double-labeled cells. Images A, B, and F–K are from longitudinal sections through 28 dpi spinal cords, other images are of transverse sections. Sections are counterstained with Hoechst dye (Hst, blue). Scale bar, 20 μm.
Similar articles
- Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes.
Hackett AR, Yahn SL, Lyapichev K, Dajnoki A, Lee DH, Rodriguez M, Cammer N, Pak J, Mehta ST, Bodamer O, Lemmon VP, Lee JK. Hackett AR, et al. Exp Neurol. 2018 Oct;308:72-79. doi: 10.1016/j.expneurol.2018.07.001. Epub 2018 Jul 3. Exp Neurol. 2018. PMID: 30008424 Free PMC article. - NG2-glia, More Than Progenitor Cells.
Eugenín-von Bernhardi J, Dimou L. Eugenín-von Bernhardi J, et al. Adv Exp Med Biol. 2016;949:27-45. doi: 10.1007/978-3-319-40764-7_2. Adv Exp Med Biol. 2016. PMID: 27714683 Review. - PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice.
Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. Rivers LE, et al. Nat Neurosci. 2008 Dec;11(12):1392-401. doi: 10.1038/nn.2220. Epub 2008 Oct 8. Nat Neurosci. 2008. PMID: 18849983 Free PMC article. - The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS.
Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J. Reynolds R, et al. J Neurocytol. 2002 Jul-Aug;31(6-7):523-36. doi: 10.1023/a:1025747832215. J Neurocytol. 2002. PMID: 14501221 - NG2 cells in the brain: a novel glial cell population.
Nishiyama A. Nishiyama A. Hum Cell. 2001 Mar;14(1):77-82. Hum Cell. 2001. PMID: 11436356 Review.
Cited by
- Evaluating Tissue-Specific Recombination in a Pdgfrα-CreERT2 Transgenic Mouse Line.
O'Rourke M, Cullen CL, Auderset L, Pitman KA, Achatz D, Gasperini R, Young KM. O'Rourke M, et al. PLoS One. 2016 Sep 14;11(9):e0162858. doi: 10.1371/journal.pone.0162858. eCollection 2016. PLoS One. 2016. PMID: 27626928 Free PMC article. - Olig2-dependent developmental fate switch of NG2 cells.
Zhu X, Zuo H, Maher BJ, Serwanski DR, LoTurco JJ, Lu QR, Nishiyama A. Zhu X, et al. Development. 2012 Jul;139(13):2299-307. doi: 10.1242/dev.078873. Epub 2012 May 23. Development. 2012. PMID: 22627280 Free PMC article. - TREM2 mediates physical exercise-promoted neural functional recovery in rats with ischemic stroke via microglia-promoted white matter repair.
Xu J, Zhang L, Li M, He X, Luo J, Wu R, Hong Z, Zheng H, Hu X. Xu J, et al. J Neuroinflammation. 2023 Feb 25;20(1):50. doi: 10.1186/s12974-023-02741-w. J Neuroinflammation. 2023. PMID: 36829205 Free PMC article. - Sequential Contribution of Parenchymal and Neural Stem Cell-Derived Oligodendrocyte Precursor Cells toward Remyelination.
Serwanski DR, Rasmussen AL, Brunquell CB, Perkins SS, Nishiyama A. Serwanski DR, et al. Neuroglia. 2018 Sep;1(1):91-105. doi: 10.3390/neuroglia1010008. Epub 2018 Jun 12. Neuroglia. 2018. PMID: 30662979 Free PMC article. - Nestin-dependent mitochondria-ER contacts define stem Leydig cell differentiation to attenuate male reproductive ageing.
Yao S, Wei X, Deng W, Wang B, Cai J, Huang Y, Lai X, Qiu Y, Wang Y, Guan Y, Wang J. Yao S, et al. Nat Commun. 2022 Jul 11;13(1):4020. doi: 10.1038/s41467-022-31755-w. Nat Commun. 2022. PMID: 35821241 Free PMC article.
References
- Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. - PubMed
- Butt AM, Hornby MF, Ibrahim M, Kirvell S, Graham A, Berry M. PDGF-alpha receptor and myelin basic protein mRNAs are not coexpressed by oligodendrocytes in vivo: a double in situ hybridization study in the anterior medullary velum of the neonatal rat. Mol Cell Neurosci. 1997;8:311–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0800575(86419)/MRC_/Medical Research Council/United Kingdom
- G0800575/MRC_/Medical Research Council/United Kingdom
- Wellcome Trust/United Kingdom
- 080513/Wellcome Trust/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous