The Lkb1 metabolic sensor maintains haematopoietic stem cell survival - PubMed (original) (raw)
. 2010 Dec 2;468(7324):659-63.
doi: 10.1038/nature09572.
Stephanie Z Xie, Brinda Alagesan, Judith Kim, Rushdia Z Yusuf, Borja Saez, Alexandros Tzatsos, Fatih Ozsolak, Patrice Milos, Francesco Ferrari, Peter J Park, Orian S Shirihai, David T Scadden, Nabeel Bardeesy
Affiliations
- PMID: 21124451
- PMCID: PMC3037591
- DOI: 10.1038/nature09572
The Lkb1 metabolic sensor maintains haematopoietic stem cell survival
Sushma Gurumurthy et al. Nature. 2010.
Erratum in
- Nature. 2011 Aug 4;476(7358):114
Abstract
Haematopoietic stem cells (HSCs) can convert between growth states that have marked differences in bioenergetic needs. Although often quiescent in adults, these cells become proliferative upon physiological demand. Balancing HSC energetics in response to nutrient availability and growth state is poorly understood, yet essential for the dynamism of the haematopoietic system. Here we show that the Lkb1 tumour suppressor is critical for the maintenance of energy homeostasis in haematopoietic cells. Lkb1 inactivation in adult mice causes loss of HSC quiescence followed by rapid depletion of all haematopoietic subpopulations. Lkb1-deficient bone marrow cells exhibit mitochondrial defects, alterations in lipid and nucleotide metabolism, and depletion of cellular ATP. The haematopoietic effects are largely independent of Lkb1 regulation of AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling. Instead, these data define a central role for Lkb1 in restricting HSC entry into cell cycle and in broadly maintaining energy homeostasis in haematopoietic cells through a novel metabolic checkpoint.
Figures
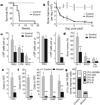
Figure 1. Lkb1 is required for haematopoiesis
Mutant (Mx1-cre; Lkb1L/L) and control (_Mx1-cre; Lkb1_L/+ or Lkb1L/L) mice were injected with pIpC every second day over 7 days. a, Survival analysis; n >10 mice per genotype; P<0.001. b, Mononuclear bone marrow cellularity (_n_=4). c–f, Analysis at day 5 for the indicated subpopulations (n = 6 mice per genotype). CLP, common lymphoid progenitors; CMP, common myeloid progenitors; GMP, granulocyte-macrophage progenitors; MEP, megakaryocyte-erythrocyte progenitors; NEU, neutrophil; RBC, red blood cell. g, CFU-C assay for myeloid progenitors. *P < 0.01; **P < 0.001. Error bars in b–f indicate mean ± s.d.
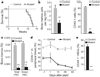
Figure 2. Cell-autonomous role of Lkb1 in haematopoiesis
a–c, After pIpC induction, transplanted mice were analysed for survival (P < 0.005) (**a**), bone marrow cellularity (left) and donor chimaerism (right) on day 5 (**b**), and total HSC numbers and donor contribution at day 18 (**c**). **d, e**, Competitively transplanted mice were induced with pIpC and analysed for CD45.1 status in peripheral blood (**d**), and per cent CD45.1 HSCs at 4 weeks (**e**). _n_ > 6 mice per genotype in a–e. *P < 0.05, **P < 0.01, all error bars indicate mean ± s.d.
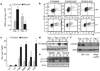
Figure 3. Impact of Lkb1 inactivation on proliferation and apoptosis
a, Quantification of HSCs in the Mx1-cre model at day −3 and day +2 after pIpC treatment. n = 3 mice per genotype; P < 0.01, error bars indicate mean ± s.d. b, Cell-cycle analysis of HSCs (Ki-67/propidium iodide (PI) staining) at day −3 (n = 3; P < 0.01). c, Analysis viability in HSC and progenitor cells and Lin+ cells at day 5 by 7AAD staining (*P < 0.001, error bars indicate mean ± s.d.). d–f, Immunoblot of control (C) and Lkb1 mutant (M) mice for cleaved caspase-3 (Cl.Casp3) in bone marrow (BM) (d), LC3 in the indicated tissues at day 5 after pIpC (e), and phospho-H2AX levels in bone marrow (f).
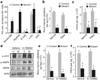
Figure 4. mTORC1 inhibition and AMPK activation do not rescue bone marrow failure in Lkb1 mutants
a, Phospho(Ser 235/236)-S6 expression in bone marrow subpopulations at day 5 after pIpC treatment. b, c, Rapamycin (Rapa.) treatment does not rescue the drop in bone marrow cellularity (b) or HSCs (c) in Lkb1 mutants at day 5 (n = 4 mice per genotype). d–f, The AMPK activator A-769662 restores phospho(Ser 79)-ACC levels in Lkb1 mutant bone marrow cells (d), yet does not rescue loss of bone marrow cellularity (e), or HSCs (f) at day 5 (n = 4). * P < 0.001, all error bars indicate mean ± s.d.
Figure 5. Inactivation of Lkb1 alters mitochondrial function of bone marrow cells
a, Mitochondrial membrane potential of control and mutant cells at day 3 after pIpC assayed by DilC5 staining. b, Oxygen consumption rates (OCR) in control and mutant bone marrow cells under basal conditions and in response to 0.25 µM oligomycin, 5 µM fluoro-carbonyl cyanide phenylhydrazone (FCCP) or 1 µM antimycin + rotenone at day 1 in the Rosa26-creERt2 (top) and Mx1-cre (bottom) models. c, ATP levels of bone marrow cells at day 5 after pIpC. d, Glucose uptake in bone marrow at day 1. NBDG, fluorescent
d
-glucose analogue. e, Quantification of relative mitochondrial mass by Mitotracker staining (Mx1-cre model). n = 3 mice per genotype; P < 0.001; all error bars indicate mean ± s.d.
Comment in
- Stem cells: The blood balance.
Durand EM, Zon LI. Durand EM, et al. Nature. 2010 Dec 2;468(7324):644-5. doi: 10.1038/468644a. Nature. 2010. PMID: 21124447 No abstract available. - Stem cells: LKB1 maintains the balance.
David R. David R. Nat Rev Mol Cell Biol. 2011 Jan;12(1):4. doi: 10.1038/nrm3032. Epub 2010 Dec 8. Nat Rev Mol Cell Biol. 2011. PMID: 21139635 No abstract available. - The tumor suppressor LKB1 emerges as a critical factor in hematopoietic stem cell biology.
Krock B, Skuli N, Simon MC. Krock B, et al. Cell Metab. 2011 Jan 5;13(1):8-10. doi: 10.1016/j.cmet.2010.12.015. Cell Metab. 2011. PMID: 21195344
Similar articles
- Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells.
Nakada D, Saunders TL, Morrison SJ. Nakada D, et al. Nature. 2010 Dec 2;468(7324):653-8. doi: 10.1038/nature09571. Nature. 2010. PMID: 21124450 Free PMC article. - Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells.
Gan B, Hu J, Jiang S, Liu Y, Sahin E, Zhuang L, Fletcher-Sananikone E, Colla S, Wang YA, Chin L, Depinho RA. Gan B, et al. Nature. 2010 Dec 2;468(7324):701-4. doi: 10.1038/nature09595. Nature. 2010. PMID: 21124456 Free PMC article. - Stem cells: The blood balance.
Durand EM, Zon LI. Durand EM, et al. Nature. 2010 Dec 2;468(7324):644-5. doi: 10.1038/468644a. Nature. 2010. PMID: 21124447 No abstract available. - LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.
Shaw RJ. Shaw RJ. Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review. - mTORC signaling in hematopoiesis.
Wang X, Chu Y, Wang W, Yuan W. Wang X, et al. Int J Hematol. 2016 May;103(5):510-8. doi: 10.1007/s12185-016-1944-z. Epub 2016 Jan 20. Int J Hematol. 2016. PMID: 26791377 Review.
Cited by
- Staying alive: metabolic adaptations to quiescence.
Valcourt JR, Lemons JM, Haley EM, Kojima M, Demuren OO, Coller HA. Valcourt JR, et al. Cell Cycle. 2012 May 1;11(9):1680-96. doi: 10.4161/cc.19879. Epub 2012 May 1. Cell Cycle. 2012. PMID: 22510571 Free PMC article. Review. - Association of Myeloid Liver Kinase B1 Depletion With a Reduction in Alveolar Macrophage Numbers and an Impaired Host Defense During Gram-Negative Pneumonia.
Otto NA, de Vos AF, van Heijst JWJ, Roelofs JJTH, van der Poll T. Otto NA, et al. J Infect Dis. 2022 Apr 1;225(7):1284-1295. doi: 10.1093/infdis/jiaa416. J Infect Dis. 2022. PMID: 32648919 Free PMC article. - Multifaceted roles of GSK-3 and Wnt/β-catenin in hematopoiesis and leukemogenesis: opportunities for therapeutic intervention.
McCubrey JA, Steelman LS, Bertrand FE, Davis NM, Abrams SL, Montalto G, D'Assoro AB, Libra M, Nicoletti F, Maestro R, Basecke J, Cocco L, Cervello M, Martelli AM. McCubrey JA, et al. Leukemia. 2014 Jan;28(1):15-33. doi: 10.1038/leu.2013.184. Epub 2013 Jun 19. Leukemia. 2014. PMID: 23778311 Free PMC article. Review. - Newly Identified Roles of PML in Stem Cell Biology.
Ito K, Ito K. Ito K, et al. Front Oncol. 2013 Mar 14;3:50. doi: 10.3389/fonc.2013.00050. eCollection 2013. Front Oncol. 2013. PMID: 23504288 Free PMC article. - Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity.
Latil M, Rocheteau P, Châtre L, Sanulli S, Mémet S, Ricchetti M, Tajbakhsh S, Chrétien F. Latil M, et al. Nat Commun. 2012 Jun 12;3:903. doi: 10.1038/ncomms1890. Nat Commun. 2012. PMID: 22692546
References
- Tothova Z, Gilliland DG. FoxO transcription factors and stemcell homeostasis: insights from the hematopoietic system. Cell Stem Cell. 2007;1:140–152. - PubMed
- Ito K, et al. Regulation of reactive oxygen species by Atm is essential for proper response toDNA double-strand breaks in lymphocytes. J. Immunol. 2007;178:103–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK050234-13/DK/NIDDK NIH HHS/United States
- R01 HG005230-01/HG/NHGRI NIH HHS/United States
- R01 DK050234-12/DK/NIDDK NIH HHS/United States
- U01 CA141576-01/CA/NCI NIH HHS/United States
- R01 HG005230/HG/NHGRI NIH HHS/United States
- R01 DK050234/DK/NIDDK NIH HHS/United States
- U01 CA141576/CA/NCI NIH HHS/United States
- DK050234/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous