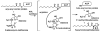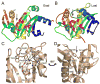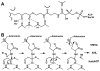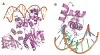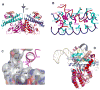Structural basis of acyl-homoserine lactone-dependent signaling - PubMed (original) (raw)
Review
. 2011 Jan 12;111(1):68-85.
doi: 10.1021/cr1000817. Epub 2010 Dec 2.
Affiliations
- PMID: 21125993
- PMCID: PMC3494288
- DOI: 10.1021/cr1000817
Review
Structural basis of acyl-homoserine lactone-dependent signaling
Mair E A Churchill et al. Chem Rev. 2011.
No abstract available
Figures
Figure 1
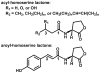
Chemical structure of homoserine lactone-based bacterial signaling molecules. The acyl-homoserine lactones (AHLs) found in Proteobacteria vary by substitution at the C3 position (R1) and the length and unsaturation at the C1 position indicated by R2. Shown also is the structure of the first aroyl-homoserine lactone, para-coumaroyl-HSL (pC-HSL).
Figure 2
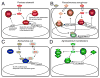
Quorum-sensing systems for which there is structural information. (A) Pantoea stewartii. EsaI constitutively produces 3-oxo-C6-HSL. EsaR binds to the AHL and is derepressed by this binding, which leads to expression of quorum sensing regulated genes in the EsaR regulon and a feedback loop regulating EsaR expression. (B) Pseudomonas aeruginosa. LasI and RhlI produce 3-oxo-C12-HSL and C4-HSL, respectively. The AHLs bind to the cognate R proteins LasR and RhlR, respectively, and activate or repress transcription of the genes in the Rhl and Las regulons. In addition, there is an orphan receptor QscR that has a regulon that overlaps with LasR. QscR can bind to AHLs made by LasI as well as exogenous AHLs that _Pseudomona_s does not make. The Las system is as at the top of the hierarchy and several feedback loops are found in this quorum sensing system. (C) Escherichia coli. E. coli does not have an AHL synthase, but it does have a LuxR homolog SdiA, which can bind to AHLs and regulate gene expression in response to exogenous AHLs. (D) Agrobacterium tumefaciens. TraI synthesizes 3-oxo-C8-HSL, which binds to TraR, leading to activation of TraR-controlled genes.
Figure 3
Schematic diagram of the reaction performed by AHL synthase enzymes. AHL synthases catalyze the formation of AHLs from _S_-adenosyl-L-methionine (SAM) and acyl-ACP by acylation of SAM and lactonization of the methionine moiety to give in addition to the AHL, holo-ACP, and 5’-methylthioadenosine (5’MTA) products ,.
Figure 4
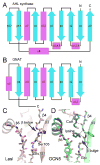
Primary structure of the AHL synthases and the pC-HSL synthase RpaI. A sequence and topology diagram shows the sequences of several AHL synthases mapped to the structural descriptions of the AHL synthases LasI and EsaI. The grey shaded regions are the most conserved sequence blocks within the AHL synthase family. The eight conserved residues of the AHL synthase family are highlighted in solid colors, and those that are similar among AHL synthases are boxed. Below the sequences are shown the alpha helices (magenta) and beta strands (cyan) observed in the structures of EsaI and LasI. The lettering ‘a’ and ‘p’ indicate the amino acid residues proposed to interact with the acyl chain or phosphopantetheine moieties of acyl-ACP, respectively.
Figure 5
Structure of the AHL synthases EsaI and LasI. (A) Ribbon diagrams depict the backbone structures of EsaI (A) and LasI (B). The structure of the EsaI enzyme (PDB ID 1kzf) from Pantoea stewartii was determined from the native sequence. The structure of the LasI enzyme (PDB ID 1ro5) from Pseudomonas aeruginosa was determined from an active form that had been engineered to improve solubility and crystallization properties. The rainbow coloring is blue to red from the N-terminus to the C-terminus and the major secondary structure elements are labeled. The most conserved residues are indicated with magenta coloring. (C) Close-up view of the LasI active site residues, showing the conserved residues and the electrostatic cluster. Well-ordered water molecules in the active site are shown as red spheres and putative hydrogen bonds are shown as dotted black lines. (D) Close-up view of the LasI acyl-chain binding pocket. The view is rotated approximately 180° about the vertical axis of the page relative to (C) and shows the residues lining the putative acyl-chain binding pocket. In each panel, the active site V-shaped cleft is indicated with a black arrow.
Figure 6
Similarity of AHL synthases to GNAT enzymes. Topology diagrams are shown for the AHL synthase (A) and GNAT (B) families. The alpha helices are in magenta and the beta strands are in cyan. The active site clefts of LasI (C) and Gcn5 (PDB ID 1qsn) (D) show common and different features. Well-ordered water molecules in the active site are shown as red spheres and putative hydrogen bonds are shown as dotted black lines.
Figure 7
Schematic diagram of the proposed AHL synthesis mechanism. (A) The interactions predicted to form between acyl-phosphopantetheine bound in the active site of a generic AHL synthase are depicted as dotted lines for hydrogen bonds and curved lines for other types of interactions, such as van der Waals contacts. (B) The proposed mechanism of acylation involves a direct nucleophilic attack by the amine of SAM on the C1 position of the acyl chain, which will eventually release holo-ACP. The mechanism of lactonization is a direct nucleophilic attack on the Cγ position of SAM by the carboxylate oxygen atom, which will produce the AHL and release the product 5’MTA.
Figure 8
Structural comparison of the LuxR-type proteins. A) Conformations of AHLs in the AHL-binding pockets: 3-oxo-C8-HSL (green) in TraRAt (pink, PDB ID: 1L3L); 3-oxo-C12-HSL (cyan) in LasR (light blue, PDB ID: 2UV0); C8-HSL (yellow) in SdiA (gray, PDB ID: 2AVX). For clarity, structural elements (helices and loops) that are located on the top of the bound AHLs are removed. The different orientations of the 3-oxo group in 3-oxo-C8-HSL and 3-oxo-C12-HSL that lead to drastic conformations of the acyl chains are highlighted with arrows. B) and C) Distinct dimeric assembly of TraR and LasR shown in the same view. Structure of dimeric TraRAt NTD (pink) is overlaid with that of LasR NTD (light blue) by superimposing one (left) NTD of each dimer. The main helices that are located in the TraRAt dimer interface are largely parallel and highlighted in red, and those in LasR are almost perpendicular and in green. The dimer contacts provided by loops of α4-β3 and β4-β5 in LasR are highlighted in dotted circles. D) and E) Surface electrostatic potentials of TraRAt and SdiA, respectively. Surface of one TraRAt NTD is shown, the other NTD is shown as a Cα trace in magenta. The view of SdiA corresponds to that with the surface presentation of TraRAt monomer. Surface with positive potentials is colored in blue, and with negative potentials in red.
Figure 9
A) The overall structure of the TraRAt-DNA complex (PDB ID: 1L3L). Two TraR monomers (purple and pink) adopt an asymmetric relationship when bound to DNA. The DNA recognition helix α12 projects into the DNA major groove. The α13 helices from each of the two subunits are in contact due to binding of CTDs to the adjacent DNA major grooves. 3-oxo-C8-HSL is shown in green, and DNA in orange. B) Decoding the DNA sequence by TraRAt α12. Side chains of three TraR residues (Tyr202, Arg206 and Arg211) that interact with bases at P3-P7 positions are shown. For clarity, only one CTD and one half-site tra box is shown.
Figure 10
Surface potentials of TraMAt (PDB ID: 1RFY) and TraMNGR (PDB ID: 2Q0O) (from the anti-activation complex). For dimeric TraMAt, surface of one monomer is shown, the other monomer is shown as a Cα trace in yellow. Surface with positive potentials is colored in blue, and with negative potentials in red. TraMNGR is shown in the same view as the surface presentation of TraMAt monomer.
Figure 11
Structure and function of anti-activation by TraM. A) Model of symmetric (TraRNGR-TraMNGR)2 in solution. The model was generated by applying the C2 rotational symmetry of the NTDs to the closed form of dimeric TraRNGR-TraMNGR. The repositioned TraRNGR-TraMNGR pair is colored in red and blue, and the original pair is in magenta and cyan. The main TraM-binding site, α10 and the linker, is in green, and the DNA binding helix, α12, is in orange. The bound 3-oxo-C8-HSL is shown in ball-and-stick representation. B) The interaction between TraRNGR α10 with TraMNGR (PDB ID: 2Q0O). TraMNGR residues (cyan) that are in contact with Pro178, Leu182 and Trp186 (magenta) of TraRNGR α10 are shown. Leu199 from α11 is also shown. The dotted lines denote hydrogen bonds between TraRNGR Trp186 and TraMNGR His39 and Gln85. C) The interaction of TraRNGR L199 with the hydrophobic C-terminal tail of TraMNGR. Surface is colored according to the underlying atoms: red for oxygen, blue for nitrogen, and gray for carbon. Side chains of the hydrophobic TraMNGR residues (gray), along with that of L199 (magenta), are shown. D) Structural comparison of TraRAt-DNA with TraRNGR-TraMNGR. The NTDs of the both structures are superimposed, but for clarity only one monomer of TraRNGR (in red) and TraRAt (in purple, the extended form) from each structure is shown. The large domain movement is highlighted with an arrow. DNA is displayed as a double coil and is light orange, and the DNA-binding helix α12 is yellow in TraRAt-DNA complex and orange in TraRNGR-TraMNGR complex. TraMNGR is light blue, and the TraM-binding site (α10 and the linker) is cyan in TraRAt-DNA and green in TraRNGR-TraMNGR.
Similar articles
- _N-_Acyl Homoserine Lactone Analog Modulators of the Pseudomonas aeruginosa Rhll Quorum Sensing Signal Synthase.
Shin D, Gorgulla C, Boursier ME, Rexrode N, Brown EC, Arthanari H, Blackwell HE, Nagarajan R. Shin D, et al. ACS Chem Biol. 2019 Oct 18;14(10):2305-2314. doi: 10.1021/acschembio.9b00671. Epub 2019 Oct 9. ACS Chem Biol. 2019. PMID: 31545595 Free PMC article. - N-Acyl Homoserine Lactone-Mediated Quorum Sensing in Aeromonas veronii biovar sobria Strain 159: Identification of LuxRI Homologs.
Chan XY, How KY, Yin WF, Chan KG. Chan XY, et al. Front Cell Infect Microbiol. 2016 Feb 16;6:7. doi: 10.3389/fcimb.2016.00007. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26909339 Free PMC article. No abstract available. - Crystal structure of QscR, a Pseudomonas aeruginosa quorum sensing signal receptor.
Lintz MJ, Oinuma K, Wysoczynski CL, Greenberg EP, Churchill ME. Lintz MJ, et al. Proc Natl Acad Sci U S A. 2011 Sep 20;108(38):15763-8. doi: 10.1073/pnas.1112398108. Epub 2011 Sep 12. Proc Natl Acad Sci U S A. 2011. PMID: 21911405 Free PMC article. - Mechanisms and synthetic modulators of AHL-dependent gene regulation.
Stevens AM, Queneau Y, Soulère L, von Bodman S, Doutheau A. Stevens AM, et al. Chem Rev. 2011 Jan 12;111(1):4-27. doi: 10.1021/cr100064s. Epub 2010 Dec 13. Chem Rev. 2011. PMID: 21142091 Review. No abstract available. - Acyl-homoserine lactone quorum sensing in the Roseobacter clade.
Zan J, Liu Y, Fuqua C, Hill RT. Zan J, et al. Int J Mol Sci. 2014 Jan 7;15(1):654-69. doi: 10.3390/ijms15010654. Int J Mol Sci. 2014. PMID: 24402124 Free PMC article. Review.
Cited by
- Quorum Sensing Inhibitors: An Alternative Strategy to Win the Battle against Multidrug-Resistant (MDR) Bacteria.
Hetta HF, Ramadan YN, Rashed ZI, Alharbi AA, Alsharef S, Alkindy TT, Alkhamali A, Albalawi AS, Battah B, Donadu MG. Hetta HF, et al. Molecules. 2024 Jul 24;29(15):3466. doi: 10.3390/molecules29153466. Molecules. 2024. PMID: 39124871 Free PMC article. Review. - The autoinducer synthases LuxI and AinS are responsible for temperature-dependent AHL production in the fish pathogen Aliivibrio salmonicida.
Hansen H, Purohit AA, Leiros HK, Johansen JA, Kellermann SJ, Bjelland AM, Willassen NP. Hansen H, et al. BMC Microbiol. 2015 Mar 24;15:69. doi: 10.1186/s12866-015-0402-z. BMC Microbiol. 2015. PMID: 25886758 Free PMC article. - Quorum sensing regulates 'swim-or-stick' lifestyle in the phycosphere.
Fei C, Ochsenkühn MA, Shibl AA, Isaac A, Wang C, Amin SA. Fei C, et al. Environ Microbiol. 2020 Nov;22(11):4761-4778. doi: 10.1111/1462-2920.15228. Epub 2020 Sep 17. Environ Microbiol. 2020. PMID: 32896070 Free PMC article. - Molecular Mechanisms and Applications of N-Acyl Homoserine Lactone-Mediated Quorum Sensing in Bacteria.
Kumar L, Patel SKS, Kharga K, Kumar R, Kumar P, Pandohee J, Kulshresha S, Harjai K, Chhibber S. Kumar L, et al. Molecules. 2022 Nov 4;27(21):7584. doi: 10.3390/molecules27217584. Molecules. 2022. PMID: 36364411 Free PMC article. Review. - Chemical methods to interrogate bacterial quorum sensing pathways.
Praneenararat T, Palmer AG, Blackwell HE. Praneenararat T, et al. Org Biomol Chem. 2012 Oct 3;10(41):8189-99. doi: 10.1039/c2ob26353j. Org Biomol Chem. 2012. PMID: 22948815 Free PMC article.
References
- Eberhard A, Burlingame AL, Eberhard C, Kenyon GL, Nealson KH, Oppenheimer NJ. Biochemistry. 1981;20:2444. - PubMed
- Nealson KH. Arch Microbiol. 1977;112:73. - PubMed
- Chemical communication among Bacteria. American Microbiology Association Press; Washington, DC: 2008.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources