Circadian integration of metabolism and energetics - PubMed (original) (raw)
Review
Circadian integration of metabolism and energetics
Joseph Bass et al. Science. 2010.
Abstract
Circadian clocks align behavioral and biochemical processes with the day/night cycle. Nearly all vertebrate cells possess self-sustained clocks that couple endogenous rhythms with changes in cellular environment. Genetic disruption of clock genes in mice perturbs metabolic functions of specific tissues at distinct phases of the sleep/wake cycle. Circadian desynchrony, a characteristic of shift work and sleep disruption in humans, also leads to metabolic pathologies. Here, we review advances in understanding the interrelationship among circadian disruption, sleep deprivation, obesity, and diabetes and implications for rational therapeutics for these conditions.
Figures
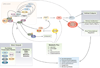
Figure 1. Central and peripheral clocks coordinate external cues with behavior and metabolic outputs
Light entrains the master pacemaker in the suprachiasmatic nucleus (SCN), which in turn synchronizes extra-SCN and peripheral clocks. Brain clock outputs include behavioral rhythms (i.e., sleep, feeding), while peripheral clock outputs include metabolic rhythms (i.e., glucose and lipid homeostasis, etc). The hierarchical organization of the mammalian clock is highlighted, with “non-autonomous” regulation of peripheral tissue clocks denoting the regulation of peripheral tissue oscillators through direct neural and humoral signals, and “autonomous” regulation indicating the intrinsic regulation of local cellular oscillators independently of the brain clock. Highlighted to the right are the three possible ways to disrupt the clock, by changing either period, phase, or amplitude, each of which can trigger disorders of metabolism. Phase resetting can be broadly classified into two groups based upon phase response following delivery of the agent at sequential time points across the 24 hr cycle. Type 1 resetting indicates that the slope of the plot relating the new to old circadian phase is 1 (interventions that cause different phase shifts at different circadian times). Type 0 resetting indicates that the slope of the new to old circadian phase is 0 (i.e., interventions that cause the same phase at all circadian times). Abbreviations: PVN, paraventricular nucleus; PIT, pituitary; ARC, arcuate nucleus; LHA, lateral hypothalamic area.
Figure 2. Direct and indirect outputs of the core clock mechanism
The core clock consists of a series of transcription/translation feedback loops that synchronize diverse metabolic processes through both direct and indirect outputs including gluconeogenesis and oxidative metabolism (see text for details). The clock also receives reciprocal input from nutrient signaling pathways (including SIRT1 and AMPK), which function as rheostats to couple circadian cycles to metabolic flux especially in peripheral tissues.
Figure 3. The clock partitions behavioral and metabolic processes according to time of day
The clock coordinates appropriate metabolic responses within peripheral tissues with the light-dark cycle. For example, the liver clock promotes gluconeogenesis and glycogenolysis during the sleep/fasting period, while it promotes glycogen and cholesterol synthesis during the wake/feeding period. Proper functioning of peripheral clocks keeps metabolic processes in sync with the environment, which is critical for maintaining health of the organism. Different tissues exhibit distinct clock-controlled properties thus ablation of the clock in certain tissues will cause opposing effects on metabolic function as uncovered through dynamic challenges at different times in the cycle under different nutrient conditions. Aging, diet, and environmental disruption such as shift-work may also impact the integration of circadian and metabolic systems.
Similar articles
- Misalignment of Circadian Rhythms in Diet-Induced Obesity.
Engin A. Engin A. Adv Exp Med Biol. 2024;1460:27-71. doi: 10.1007/978-3-031-63657-8_2. Adv Exp Med Biol. 2024. PMID: 39287848 Review. - Circadian rhythms, sleep, and metabolism.
Huang W, Ramsey KM, Marcheva B, Bass J. Huang W, et al. J Clin Invest. 2011 Jun;121(6):2133-41. doi: 10.1172/JCI46043. Epub 2011 Jun 1. J Clin Invest. 2011. PMID: 21633182 Free PMC article. Review. - [Sleep/wake cycle, circadian disruption and the development of obesity].
Masaki T. Masaki T. Nihon Rinsho. 2012 Jul;70(7):1183-7. Nihon Rinsho. 2012. PMID: 22844802 Japanese. - Circadian clocks in fuel harvesting and energy homeostasis.
Ramsey KM, Bass J. Ramsey KM, et al. Cold Spring Harb Symp Quant Biol. 2011;76:63-72. doi: 10.1101/sqb.2011.76.010546. Epub 2011 Sep 2. Cold Spring Harb Symp Quant Biol. 2011. PMID: 21890641 Free PMC article. Review. - Impact of Sleep and Circadian Disruption on Energy Balance and Diabetes: A Summary of Workshop Discussions.
Arble DM, Bass J, Behn CD, Butler MP, Challet E, Czeisler C, Depner CM, Elmquist J, Franken P, Grandner MA, Hanlon EC, Keene AC, Joyner MJ, Karatsoreos I, Kern PA, Klein S, Morris CJ, Pack AI, Panda S, Ptacek LJ, Punjabi NM, Sassone-Corsi P, Scheer FA, Saxena R, Seaquest ER, Thimgan MS, Van Cauter E, Wright KP. Arble DM, et al. Sleep. 2015 Dec 1;38(12):1849-60. doi: 10.5665/sleep.5226. Sleep. 2015. PMID: 26564131 Free PMC article.
Cited by
- RNA expression profiling in depressed patients suggests retinoid-related orphan receptor alpha as a biomarker for antidepressant response.
Hennings JM, Uhr M, Klengel T, Weber P, Pütz B, Touma C, Czamara D, Ising M, Holsboer F, Lucae S. Hennings JM, et al. Transl Psychiatry. 2015 Mar 31;5(3):e538. doi: 10.1038/tp.2015.9. Transl Psychiatry. 2015. PMID: 25826113 Free PMC article. - In vivo single-cell detection of metabolic oscillations in stem cells.
Stringari C, Wang H, Geyfman M, Crosignani V, Kumar V, Takahashi JS, Andersen B, Gratton E. Stringari C, et al. Cell Rep. 2015 Jan 6;10(1):1-7. doi: 10.1016/j.celrep.2014.12.007. Epub 2014 Dec 24. Cell Rep. 2015. PMID: 25543138 Free PMC article. - Human peripheral clocks: applications for studying circadian phenotypes in physiology and pathophysiology.
Saini C, Brown SA, Dibner C. Saini C, et al. Front Neurol. 2015 May 13;6:95. doi: 10.3389/fneur.2015.00095. eCollection 2015. Front Neurol. 2015. PMID: 26029154 Free PMC article. Review. - The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways.
Nam D, Guo B, Chatterjee S, Chen MH, Nelson D, Yechoor VK, Ma K. Nam D, et al. J Cell Sci. 2015 May 1;128(9):1835-47. doi: 10.1242/jcs.167643. Epub 2015 Mar 6. J Cell Sci. 2015. PMID: 25749863 Free PMC article. - The skeletal muscle circadian clock regulates titin splicing through RBM20.
Riley LA, Zhang X, Douglas CM, Mijares JM, Hammers DW, Wolff CA, Wood NB, Olafson HR, Du P, Labeit S, Previs MJ, Wang ET, Esser KA. Riley LA, et al. Elife. 2022 Sep 1;11:e76478. doi: 10.7554/eLife.76478. Elife. 2022. PMID: 36047761 Free PMC article.
References
- Rutter J, Reick M, McKnight SL. Annu Rev Biochem. 2002;71:307. - PubMed
- Nakajima M, et al. Science. 2005;308:414. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH078024/MH/NIMH NIH HHS/United States
- R01HL097817/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 HL097817/HL/NHLBI NIH HHS/United States
- P50 MH074924/MH/NIMH NIH HHS/United States
- P01 AG011412/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources