Mechanics and contraction dynamics of single platelets and implications for clot stiffening - PubMed (original) (raw)
Mechanics and contraction dynamics of single platelets and implications for clot stiffening
Wilbur A Lam et al. Nat Mater. 2011 Jan.
Abstract
Platelets interact with fibrin polymers to form blood clots at sites of vascular injury. Bulk studies have shown clots to be active materials, with platelet contraction driving the retraction and stiffening of clots. However, neither the dynamics of single-platelet contraction nor the strength and elasticity of individual platelets, both of which are important for understanding clot material properties, have been directly measured. Here we use atomic force microscopy to measure the mechanics and dynamics of single platelets. We find that platelets contract nearly instantaneously when activated by contact with fibrinogen and complete contraction within 15 min. Individual platelets can generate an average maximum contractile force of 29 nN and form adhesions stronger than 70 nN. Our measurements show that when exposed to stiffer microenvironments, platelets generated higher stall forces, which indicates that platelets may be able to contract heterogeneous clots more uniformly. The high elasticity of individual platelets, measured to be 10 kPa after contraction, combined with their high contractile forces, indicates that clots may be stiffened through direct reinforcement by platelets as well as by strain stiffening of fibrin under tension due to platelet contraction. These results show how the mechanosensitivity and mechanics of single cells can be used to dynamically alter the material properties of physiologic systems.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
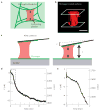
Figure 1. Measuring the contraction of single platelets with AFM
a, Cartoon of platelet contraction in a blood clot. The blood clot consists of fibrin gel, single contracting platelets, and platelet aggregates (not shown). Activated platelets adhere to fibrin polymers and contract, driving contraction of the blood clot as a whole. b, Three-dimensional isosurface rendering of multiple confocal microscopy planes shows a single thrombin-activated, membrane fluorescently labelled platelet, attached and spread between a fibrinogen-coated cantilever and a fibrinogen-coated glass surface, simulating the experimental set-up of the side-view AFM system. Scale bar = 1 μm. c, Cartoon of the experimental set-up used to measure single-platelet contraction. A fibrinogen-coated AFM cantilever is pressed slightly against an activated platelet just as it lands on a fibrinogen-coated surface. Contraction of the platelet then pulls the cantilever down towards the surface to a length, L, until the force of the cantilever, F, stalls further contraction. d, Force and platelet length measurement during a typical experiment. Tensional force, or force against platelet contraction due to the cantilever deflection towards the surface, is negative here. e, Zoom in of the first two minutes of the experiment from d showing a small compressional force applied to the platelet to initiate contact, and instantaneous contraction of the platelet.
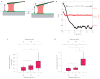
Figure 2. Stiffness dependence and timescale of platelet contraction
a, Cartoon of isometric clamp experiments that were used to simulate an infinitely stiff environment. As the platelet contracts, the surface is retracted such that the length of the platelet remains constant. b, Typical isometric experiment measurement with the cantilever deflection shown in black and platelet height shown in red. The isometric clamp is turned on after ~2 min. c,d, Distribution of stall forces and contraction rates for platelets pulling against cantilevers with stiffnesses of 18 and 43 pN nm−1, and in an isometric clamp. Medians, quartiles and 90/10 levels are shown, and the * represents a significant difference with a P value of <0.05, ** represents a significant difference with a P value of <0.01, and **** represents a significant difference with a P value of <0.001.
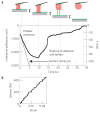
Figure 3. Elasticity and adhesion measurement for contracted platelets
a, Typical elasticity and adhesion measurement. The surface (solid grey line) is ramped out at a speed of 500 nm s−1, or 1,000 nm s−1 for a platelet for which contraction has stalled. The cantilever is pulled down until adhesion to the surface or the cantilever is ruptured, giving the adhesion force. Extensibility is defined as the length of the platelet at the rupture point, relative to its length at the beginning of the ramp-out experiment. b, Stress versus strain during the first 0.5 s of the ramp-out experiment in a. These data are fitted with a line to calculate the elasticity of the contracted platelet. Average measured elasticity was 9.85 kPA (n = 12), average extensibility was 1.57 (n = 11) and adhesion force was 69 nN (n = 11).
Figure 4. Proposed effects of platelets on clot retraction and mechanics
a, During the initial formation of a clot, activated platelets are interspersed in what is probably a heterogeneous fibrin gel. Areas of higher density of fibrin are indicated with darker shading, and exhibit higher stiffnesses. b, The stiffness dependence of platelet contraction probably results in increased forces of contraction in areas of higher stiffness (that is, higher fibrin density), possibly leading to a more uniform contraction of the clot as a whole and an increase in overall elasticity. c, We suggest that the high elasticity of contracted platelets and large adhesion forces between the platelets and the fibrin gel may allow for platelets to reinforce the mechanical properties of the clot directly, by acting as a multi-point crosslinker, restricting deformation and flow of the fibrin gel around the platelet, and by bearing some of the load. Tension on fibrin fibres due to large forces of platelet contraction also may lead to stress stiffening of the fibres under tension, further contributing to stiffening of the clot as a whole.
Comment in
- Cell mechanics: Contracting to stiffness.
Ehrlicher A, Hartwig JH. Ehrlicher A, et al. Nat Mater. 2011 Jan;10(1):12-3. doi: 10.1038/nmat2928. Nat Mater. 2011. PMID: 21157494 No abstract available.
Similar articles
- Interplay of Platelet Contractility and Elasticity of Fibrin/Erythrocytes in Blood Clot Retraction.
Tutwiler V, Wang H, Litvinov RI, Weisel JW, Shenoy VB. Tutwiler V, et al. Biophys J. 2017 Feb 28;112(4):714-723. doi: 10.1016/j.bpj.2017.01.005. Biophys J. 2017. PMID: 28256231 Free PMC article. - Recombinant fibrinogen reveals the differential roles of α- and γ-chain cross-linking and molecular heterogeneity in fibrin clot strain-stiffening.
Piechocka IK, Kurniawan NA, Grimbergen J, Koopman J, Koenderink GH. Piechocka IK, et al. J Thromb Haemost. 2017 May;15(5):938-949. doi: 10.1111/jth.13650. Epub 2017 Mar 6. J Thromb Haemost. 2017. PMID: 28166607 - Ultrastructure of clots during isometric contraction.
Cohen I, Gerrard JM, White JG. Cohen I, et al. J Cell Biol. 1982 Jun;93(3):775-87. doi: 10.1083/jcb.93.3.775. J Cell Biol. 1982. PMID: 6889599 Free PMC article. - Platelet function: its clinical significance.
Ginsburg AD, Aster RH. Ginsburg AD, et al. Dis Mon. 1970 Sep:2-42. Dis Mon. 1970. PMID: 4918554 Review. No abstract available. - Disorders of platelet function.
Bennett JS, Kolodziej MA. Bennett JS, et al. Dis Mon. 1992 Aug;38(8):577-631. Dis Mon. 1992. PMID: 1321709 Review.
Cited by
- DNA-Based Microparticle Tension Sensors (μTS) for Measuring Cell Mechanics in Non-planar Geometries and for High-Throughput Quantification.
Hu Y, Ma VP, Ma R, Chen W, Duan Y, Glazier R, Petrich BG, Li R, Salaita K. Hu Y, et al. Angew Chem Int Ed Engl. 2021 Aug 9;60(33):18044-18050. doi: 10.1002/anie.202102206. Epub 2021 Jun 28. Angew Chem Int Ed Engl. 2021. PMID: 33979471 Free PMC article. - Nonmuscle Myosin IIA Regulates Platelet Contractile Forces Through Rho Kinase and Myosin Light-Chain Kinase.
Feghhi S, Tooley WW, Sniadecki NJ. Feghhi S, et al. J Biomech Eng. 2016 Oct 1;138(10):1045061-4. doi: 10.1115/1.4034489. J Biomech Eng. 2016. PMID: 27548633 Free PMC article. - Establishment of an Experimental Intracerebral Haemorrhage Model for Mass Effect Research using a Thermo-sensitive Hydrogel.
Gong Y, Gong Y, Hou Z, Guo T, Deng J, Hao S, Wang B. Gong Y, et al. Sci Rep. 2019 Sep 25;9(1):13838. doi: 10.1038/s41598-019-50188-y. Sci Rep. 2019. PMID: 31554852 Free PMC article. - Platelet-like particles dynamically stiffen fibrin matrices and improve wound healing outcomes.
Nandi S , Sproul EP , Nellenbach K , Erb M , Gaffney L , Freytes DO , Brown AC . Nandi S , et al. Biomater Sci. 2019 Jan 29;7(2):669-682. doi: 10.1039/c8bm01201f. Biomater Sci. 2019. PMID: 30608063 Free PMC article. - Quantification of Platelet Contractile Movements during Thrombus Formation.
Tunströmer K, Faxälv L, Boknäs N, Lindahl TL. Tunströmer K, et al. Thromb Haemost. 2018 Sep;118(9):1600-1611. doi: 10.1055/s-0038-1668151. Epub 2018 Aug 15. Thromb Haemost. 2018. PMID: 30112750 Free PMC article.
References
- Hartwig JH. In: Platelets. 2. Michelson AD, editor. Elsevier; 2007. pp. 75–97.
- Jen CJ, McIntire LV. The structural properties and contractile force of a clot. Cell Motil. 1982;2:445–455. - PubMed
- Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08-HL093360/HL/NHLBI NIH HHS/United States
- R01 GM074751-03/GM/NIGMS NIH HHS/United States
- R01 GM074751/GM/NIGMS NIH HHS/United States
- R01 GM074751-02S1/GM/NIGMS NIH HHS/United States
- K08 HL093360/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources