PINK1-parkin-dependent mitophagy involves ubiquitination of mitofusins 1 and 2: Implications for Parkinson disease pathogenesis - PubMed (original) (raw)
PINK1-parkin-dependent mitophagy involves ubiquitination of mitofusins 1 and 2: Implications for Parkinson disease pathogenesis
Matthew E Gegg et al. Autophagy. 2011 Feb.
Abstract
Mitochondrial dysfunction has long been implicated in the pathogenesis of Parkinson disease (PD). Recent research has highlighted that two proteins encoded by genes linked to familial PD, PINK1 and parkin, play a role in the autophagic degradation of dysfunctional mitochondria (mitophagy). We have recently shown that mitochondrial dysfunction in PINK1-deficient human dopaminergic cells correlates with decreased autophagic flux and can be rescued by parkin expression. Further dissection of PINK1-parkin-dependent mitophagy indicates that the ubiquitination of mitofusins 1 and 2 is an early event. Here, we discuss how ubiquitination of the mitofusins might facilitate mitochondria degradation and the potential for activating mitophagy as a treatment for diseases affecting brain and muscle.
Figures
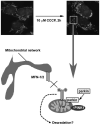
Figure 1
Immunofluorescent staining of mitochondria with an antibody against the mitochondrial protein MTCO 1 (white) in dopaminergic SH-SY5Y neuroblastoma cells indicates that the mitochondrial network undergoes fission within two hours of depolarization of mitochondria by the mitochondrial toxin CCCP. Depolarized mitochondria (↓ Ψm) display an increase in PIN K1 protein levels, which recruits parkin to mitochondria. Parkin then ubiquitinates (stars) several mitochondrial proteins including MFN-1 and MFN-2. We postulate that ubiquitination prevents the refusion of depolarized/damaged mitochondria with the mitochondrial network (cross) allowing them to be segregated for degradation by mitophagy. Ubiquitination could prevent refusion by degrading the pro-fusion proteins via the proteasome or autophagy. Alternatively, ubiquitination might inhibit MFN GTP ase activity or sterically block inter-mitochondrial and mitochondria-endoplasmic reticulum tethering by the MFNs.
Similar articles
- Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy.
Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Gegg ME, et al. Hum Mol Genet. 2010 Dec 15;19(24):4861-70. doi: 10.1093/hmg/ddq419. Epub 2010 Sep 24. Hum Mol Genet. 2010. PMID: 20871098 Free PMC article. - N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.
Eldeeb MA, Ragheb MA. Eldeeb MA, et al. Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review. - How could Parkin-mediated ubiquitination of mitofusin promote mitophagy?
Ziviani E, Whitworth AJ. Ziviani E, et al. Autophagy. 2010 Jul;6(5):660-2. doi: 10.4161/auto.6.5.12242. Epub 2010 Jul 1. Autophagy. 2010. PMID: 20484985 Free PMC article. - The PINK1-Parkin mitophagy signalling pathway is not functional in peripheral blood mononuclear cells.
Bradshaw AV, Campbell P, Schapira AHV, Morris HR, Taanman JW. Bradshaw AV, et al. PLoS One. 2021 Nov 11;16(11):e0259903. doi: 10.1371/journal.pone.0259903. eCollection 2021. PLoS One. 2021. PMID: 34762687 Free PMC article. - The PINK1-Parkin axis: An Overview.
Tanaka K. Tanaka K. Neurosci Res. 2020 Oct;159:9-15. doi: 10.1016/j.neures.2020.01.006. Epub 2020 Jan 23. Neurosci Res. 2020. PMID: 31982458 Review.
Cited by
- Exogenous Alpha-Synuclein Evoked Parkin Downregulation Promotes Mitochondrial Dysfunction in Neuronal Cells. Implications for Parkinson's Disease Pathology.
Wilkaniec A, Lenkiewicz AM, Babiec L, Murawska E, Jęśko HM, Cieślik M, Culmsee C, Adamczyk A. Wilkaniec A, et al. Front Aging Neurosci. 2021 Feb 24;13:591475. doi: 10.3389/fnagi.2021.591475. eCollection 2021. Front Aging Neurosci. 2021. PMID: 33716707 Free PMC article. - Heme oxygenase-1 regulates mitochondrial quality control in the heart.
Hull TD, Boddu R, Guo L, Tisher CC, Traylor AM, Patel B, Joseph R, Prabhu SD, Suliman HB, Piantadosi CA, Agarwal A, George JF. Hull TD, et al. JCI Insight. 2016;1(2):e85817. doi: 10.1172/jci.insight.85817. JCI Insight. 2016. PMID: 27110594 Free PMC article. - Mitochondrial and lysosomal biogenesis are activated following PINK1/parkin-mediated mitophagy.
Ivankovic D, Chau KY, Schapira AH, Gegg ME. Ivankovic D, et al. J Neurochem. 2016 Jan;136(2):388-402. doi: 10.1111/jnc.13412. Epub 2015 Nov 24. J Neurochem. 2016. PMID: 26509433 Free PMC article. - Individual Amino Acid Supplementation Can Improve Energy Metabolism and Decrease ROS Production in Neuronal Cells Overexpressing Alpha-Synuclein.
Delic V, Griffin JWD, Zivkovic S, Zhang Y, Phan TA, Gong H, Chaput D, Reynes C, Dinh VB, Cruz J, Cvitkovic E, Placides D, Frederic E, Mirzaei H, Stevens SM Jr, Jinwal U, Lee DC, Bradshaw PC. Delic V, et al. Neuromolecular Med. 2017 Sep;19(2-3):322-344. doi: 10.1007/s12017-017-8448-8. Epub 2017 Jun 15. Neuromolecular Med. 2017. PMID: 28620826 - Mitophagy and the Brain.
Swerdlow NS, Wilkins HM. Swerdlow NS, et al. Int J Mol Sci. 2020 Dec 18;21(24):9661. doi: 10.3390/ijms21249661. Int J Mol Sci. 2020. PMID: 33352896 Free PMC article. Review.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical