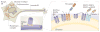Hereditary spastic paraplegias: membrane traffic and the motor pathway - PubMed (original) (raw)
Review
Hereditary spastic paraplegias: membrane traffic and the motor pathway
Craig Blackstone et al. Nat Rev Neurosci. 2011 Jan.
Erratum in
- Nat Rev Neurosci. 2011 Feb;12(2):118
Abstract
Voluntary movement is a fundamental way in which animals respond to, and interact with, their environment. In mammals, the main CNS pathway controlling voluntary movement is the corticospinal tract, which encompasses connections between the cerebral motor cortex and the spinal cord. Hereditary spastic paraplegias (HSPs) are a group of genetic disorders that lead to a length-dependent, distal axonopathy of fibres of the corticospinal tract, causing lower limb spasticity and weakness. Recent work aimed at elucidating the molecular cell biology underlying the HSPs has revealed the importance of basic cellular processes — especially membrane trafficking and organelle morphogenesis and distribution— in axonal maintenance and degeneration.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
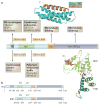
Figure 1. Spastin domain structure and interacting proteins
Domains in the spastin protein. a | The hydrophobic region (HR) possibly forms an intramembrane hairpin loop. The microtubule interacting and trafficking domain (MIT) forms a three-helix bundle that interacts with a helix in the endosomal sorting complex required for transport III (ESCRT-III) proteins charged multivesicular body protein 1B (CHMP1B) and IST1. The microtubule binding domain (MBD) is necessary for spastin to bind to microtubules and is required for microtubule severing. The AAA (ATPases associated with diverse cellular activities) ATPase domain contains the enzymatic activity of the protein that is essential for microtubule breakage. The regions to which interaction sites with known binding partners have been narrowed are indicated. The structure of the interaction between the spastin MIT domain and CHMP1B is shown, as is the structure of the Drosophila AAA ATPase domain. The amino (N)- and carboxy (C)-terminal helices are shown in magenta and blue, respectively. NBD, nucleotide binding domain; HBD, helix bundle domain; REEP1, receptor expression-enhancing protein 1. The spastin structure and interaction with CHMP1B are reproduced, with permission, from REF. © (2008) Macmillan Publishers Ltd. All rights reserved. The structure of the Drosophila AAA ATPase domain is reproduced, with permission, from REF. © (2008) Macmillan Publishers Ltd. All right reserved. b | The domain structure of the M1 spastin isoform and the M87 spastin isoform, with amino-acid numbers indicated. The position of the alternatively spliced exon 4 is shown by the dashed lines.
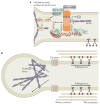
Figure 2. The spastin–Atlastin–REEP–Reticulon complex at the ER
Receptor expression-enhancing proteins (REEPs; Yop1p in yeast) and Reticulon proteins form large oligomers, referred to here as morphogen complexes, to shape the tubular endoplasmic reticulum (ER) network. Atlastin proteins (Sey1p in yeast) interact with REEPs and Reticulons and are enriched in puncta along the tubules (shown by yellow circles), including at three-way junctions. A blown-out image of the axon shows a tubular ER three-way junction. A nested blown-out image of a presumptive ER morphogen complex depicts the proposed membrane topologies for proteins involved in generating curvature of ER tubules, as well as mediating microtubule interactions and fusion of ER tubules. AAA, ATPases associated with diverse cellular activities (AAA) ATPase domain; GTP, Atlastin GTPase domain; MIT, microtubule-interacting and trafficking protein domain; MBD, microtubule-binding domain; REEP, receptor expression-enhancing protein; Rtn, reticulon.
Figure 3. The strumpellin–WAsH complex
a | The strumpellin–WASH (Wiskott–Aldrich syndrome protein and SCAR homologue) complex is recruited to endosomes by interaction between family with sequence similarity 21 (FAM21) and the vacuolar protein sorting-associated protein 35 (VPS35) component of the VPS26–VPS29–VPS35 cargo-selective retromer. Strumpellin probably interacts with the WASH complex via KIAA1033, but this remains to be proven. WASH interacts with, and activates, the actin-related protein 2 (ARP2)–ARP3 complex, which nucleates the formation of branched actin filaments. FAM21 has been proposed to interact with capping protein (CP) and promote its removal from the actin plus end, thus enhancing actin polymerization. b | In the absence of components of the WASH complex, early endosomal tubulation is enhanced, leading to the suggestion that the actin network generated by the complex is required to generate a pushing force on the tubule, which, combined with a pulling force generated by microtubule-based motors, promotes fission of the tubule by dynamin. The structure of the VPS26–VPS29–VPS35 complex is reproduced, with permission, from REF. © (2007) Macmillan Publishers Ltd. All rights reserved.
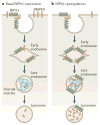
Figure 4. Model of NIPA1 action on bone morphogenetic protein receptor type-2 (BMPRII) traffic
a | In cells that express normal levels of non imprinted in Prader-Willi/Angelman syndrome 1 (NIPA1), most bone morphogenetic protein receptor type-2 (BMPRII) is situated on the plasma membrane, with small amounts in early and recycling endosomal compartments. NIPA1 is found in endosomal compartments and at the plasma membrane. Lysosomal inhibition and cellular depletion of NIPA1 both increase cellular BMPRII levels, suggesting that NIPA1 promotes degradation of BMPRII in the lysosome. b | Consistent with this idea, overexpression of NIPA1 causes dramatic internalization of BMPRII into endosomal and lysosomal compartments, accompanied by increased lysosomal degradation of BMPRII. Certain features in these diagrams are conjectural — for example, it is not known whether the initial interaction between NIPA1 and BMPRII occurs at the plasma membrane or endosomes, whether NIPA1 is degraded in lysosomes or whether it can recycle back to the plasma membrane.
Similar articles
- Cellular pathways of hereditary spastic paraplegia.
Blackstone C. Blackstone C. Annu Rev Neurosci. 2012;35:25-47. doi: 10.1146/annurev-neuro-062111-150400. Epub 2012 Apr 20. Annu Rev Neurosci. 2012. PMID: 22540978 Free PMC article. Review. - Hereditary spastic paraplegia.
Blackstone C. Blackstone C. Handb Clin Neurol. 2018;148:633-652. doi: 10.1016/B978-0-444-64076-5.00041-7. Handb Clin Neurol. 2018. PMID: 29478605 Review. - Converging cellular themes for the hereditary spastic paraplegias.
Blackstone C. Blackstone C. Curr Opin Neurobiol. 2018 Aug;51:139-146. doi: 10.1016/j.conb.2018.04.025. Epub 2018 May 10. Curr Opin Neurobiol. 2018. PMID: 29753924 Free PMC article. Review. - Traffic accidents: molecular genetic insights into the pathogenesis of the hereditary spastic paraplegias.
Soderblom C, Blackstone C. Soderblom C, et al. Pharmacol Ther. 2006 Jan;109(1-2):42-56. doi: 10.1016/j.pharmthera.2005.06.001. Epub 2005 Jul 7. Pharmacol Ther. 2006. PMID: 16005518 Review. - Axon demyelination and degeneration in a zebrafish spastizin model of hereditary spastic paraplegia.
Garg V, André S, Heyer L, Kracht G, Ruhwedel T, Scholz P, Ischebeck T, Werner HB, Dullin C, Engelmann J, Möbius W, Göpfert MC, Dosch R, Geurten BRH. Garg V, et al. Open Biol. 2024 Nov;14(11):240100. doi: 10.1098/rsob.240100. Epub 2024 Nov 6. Open Biol. 2024. PMID: 39503232 Free PMC article.
Cited by
- The hereditary spastic paraplegia protein strumpellin: characterisation in neurons and of the effect of disease mutations on WASH complex assembly and function.
Freeman C, Seaman MN, Reid E. Freeman C, et al. Biochim Biophys Acta. 2013 Jan;1832(1):160-73. doi: 10.1016/j.bbadis.2012.10.011. Epub 2012 Oct 23. Biochim Biophys Acta. 2013. PMID: 23085491 Free PMC article. - Integrating protein networks and machine learning for disease stratification in the Hereditary Spastic Paraplegias.
Vavouraki N, Tomkins JE, Kara E, Houlden H, Hardy J, Tindall MJ, Lewis PA, Manzoni C. Vavouraki N, et al. iScience. 2021 Apr 28;24(5):102484. doi: 10.1016/j.isci.2021.102484. eCollection 2021 May 21. iScience. 2021. PMID: 34113825 Free PMC article. - Vesicular dysfunction and pathways to neurodegeneration.
Lewis PA. Lewis PA. Essays Biochem. 2021 Dec 22;65(7):941-948. doi: 10.1042/EBC20210034. Essays Biochem. 2021. PMID: 34897416 Free PMC article. - Genome-wide interaction study with major depression identifies novel variants associated with cognitive function.
Thalamuthu A, Mills NT, Berger K, Minnerup H, Grotegerd D, Dannlowski U, Meinert S, Opel N, Repple J, Gruber M, Nenadić I, Stein F, Brosch K, Meller T, Pfarr JK, Forstner AJ, Hoffmann P, Nöthen MM, Witt S, Rietschel M, Kircher T, Adams M, McIntosh AM, Porteous DJ, Deary IJ, Hayward C, Campbell A, Grabe HJ, Teumer A, Homuth G, van der Auwera-Palitschka S, Schubert KO, Baune BT. Thalamuthu A, et al. Mol Psychiatry. 2022 Feb;27(2):1111-1119. doi: 10.1038/s41380-021-01379-5. Epub 2021 Nov 15. Mol Psychiatry. 2022. PMID: 34782712 Free PMC article. - Inhibiting mitochondrial fission rescues degeneration in hereditary spastic paraplegia neurons.
Chen Z, Chai E, Mou Y, Roda RH, Blackstone C, Li XJ. Chen Z, et al. Brain. 2022 Nov 21;145(11):4016-4031. doi: 10.1093/brain/awab488. Brain. 2022. PMID: 35026838 Free PMC article.
References
- Harding AE. The Hereditary Ataxias and Related Disorders. Churchill Livingston; Edinburgh: 1984.
- DeLuca GC, Ebers GC, Esiri MM. Axonal loss in multiple sclerosis: a pathological survey of the corticospinal and sensory tracts. Brain. 2004;127:1009–1018. - PubMed
- Fischer LR, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. - PubMed
- Harding AE. Hereditary spastic paraplegias. Semin Neurol. 1993;13:333–336. - PubMed
Publication types
MeSH terms
Grants and funding
- WT081386/WT_/Wellcome Trust/United Kingdom
- 082381/WT_/Wellcome Trust/United Kingdom
- MRC_/Medical Research Council/United Kingdom
- ZIA NS002992-09/ImNIH/Intramural NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases