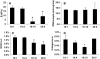Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage - PubMed (original) (raw)
Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage
Liming Bian et al. Tissue Eng Part A. 2011 Apr.
Abstract
Mesenchymal stem cells (MSCs) are being recognized as a viable cell source for cartilage repair; however, it still remains a challenge to recapitulate the functional properties of native articular cartilage using only MSCs. Additionally, MSCs may exhibit a hypertrophic phenotype under chondrogenic induction, resulting in calcification after ectopic transplantation. With this in mind, the objective of this study was to assess whether the addition of chondrocytes to MSC cultures influences the properties of tissue-engineered cartilage and MSC hypertrophy when cultured in hyaluronic acid hydrogels. Mixed cell populations (human MSCs and human chondrocytes at a ratio of 4:1) were encapsulated in the hydrogels and exhibited significantly higher Young's moduli, dynamic moduli, glycosaminoglycan levels, and collagen content than did constructs seeded with only MSCs or chondrocytes. Furthermore, the deposition of collagen X, a marker of MSC hypertrophy, was significantly lower in the coculture constructs than in the constructs seeded with MSCs alone. When MSCs and chondrocytes were cultured in distinct gels, but in the same wells, there was no improvement in biomechanical and biochemical properties of the engineered tissue, implying that a close proximity is essential. This approach can be used to improve the properties and prevent calcification of engineered cartilage formed from MSC-seeded hydrogels with the addition of lower fractions of chondrocytes, leading to improved clinical outcomes.
Figures
FIG. 1.
Cell types (human MSCs and chondrocytes) and seeding densities of experimental groups. MSCs, mesenchymal stem cells.
FIG. 2.
Viability staining of cells in HA hydrogels on days 14 and 35. Green: live cells; red: dead cells; scale bar = 100 μm. HA, hyaluronic acid. Color images available online at
.
FIG. 3.
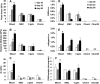
Peak stress (A), dynamic modulus (1 Hz) (B), Young's modulus (C), GAG (D), total collagen (E), and DNA (F) content of all experimental groups (data presented as mean ± standard deviation). ND: not detectable, *p < 0.05 versus all other groups at the same culture time; †p < 0.05 versus MSC at the same culture time (n = 4). The Chon20 group was not tested for mechanical properties due to poor early cell viability. GAG, glycosaminoglycan.
FIG. 4.
Gene expression (in fold change) of selected chondrogenic and hypertrophic markers with time in culture (data presented as mean ± standard deviation). Only the Mixed and MSC groups were compared due to limited viability in other experimental groups. *p < 0.05 versus MSC group (n = 3).
FIG. 5.
Immunohistochemical staining for chondroitin sulfate (CS), type II collagen, and type I collagen on day 42; bar in inset = 50 μm. Color images available online at
.
FIG. 6.
Immunohistochemical staining for collagen 10A1 on day 42; bar in inset = 25 μm. Color images available online at
.
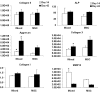
FIG. 7.
Young's modulus (A), DNA (B), GAG (C), and total collagen (D) content of all groups on day 42. The entities 19:1, 16:4, 10:10, and 20:0 represent ratios of MSCs and chondrocytes seeded in mixture in terms of million cells/mL (i.e., 19:1 indicates a seeding density of 19 million MSCs and 1 million chondrocytes per mL of hydrogel and cell mixture, data presented as mean ± standard deviation). *p < 0.05 versus 19:1 and 16:4 group (n = 4).
Similar articles
- Cartilage matrix formation by bovine mesenchymal stem cells in three-dimensional culture is age-dependent.
Erickson IE, van Veen SC, Sengupta S, Kestle SR, Mauck RL. Erickson IE, et al. Clin Orthop Relat Res. 2011 Oct;469(10):2744-53. doi: 10.1007/s11999-011-1869-z. Clin Orthop Relat Res. 2011. PMID: 21424832 Free PMC article. - Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels.
Bian L, Zhai DY, Zhang EC, Mauck RL, Burdick JA. Bian L, et al. Tissue Eng Part A. 2012 Apr;18(7-8):715-24. doi: 10.1089/ten.TEA.2011.0455. Epub 2011 Dec 2. Tissue Eng Part A. 2012. PMID: 21988555 Free PMC article. - Synergistic effects on mesenchymal stem cell-based cartilage regeneration by chondrogenic preconditioning and mechanical stimulation.
Lin S, Lee WYW, Feng Q, Xu L, Wang B, Man GCW, Chen Y, Jiang X, Bian L, Cui L, Wei B, Li G. Lin S, et al. Stem Cell Res Ther. 2017 Oct 3;8(1):221. doi: 10.1186/s13287-017-0672-5. Stem Cell Res Ther. 2017. PMID: 28974254 Free PMC article. - Enhancing chondrogenic phenotype for cartilage tissue engineering: monoculture and coculture of articular chondrocytes and mesenchymal stem cells.
Hubka KM, Dahlin RL, Meretoja VV, Kasper FK, Mikos AG. Hubka KM, et al. Tissue Eng Part B Rev. 2014 Dec;20(6):641-54. doi: 10.1089/ten.TEB.2014.0034. Epub 2014 Jun 23. Tissue Eng Part B Rev. 2014. PMID: 24834484 Free PMC article. Review. - The use of mesenchymal stem cells for chondrogenesis.
Pelttari K, Steck E, Richter W. Pelttari K, et al. Injury. 2008 Apr;39 Suppl 1:S58-65. doi: 10.1016/j.injury.2008.01.038. Injury. 2008. PMID: 18313473 Review.
Cited by
- Cellular and Acellular Approaches for Cartilage Repair: A Philosophical Analysis.
Brittberg M. Brittberg M. Cartilage. 2015 Apr;6(2 Suppl):4S-12S. doi: 10.1177/1947603514536983. Epub 2015 Mar 24. Cartilage. 2015. PMID: 27340516 Free PMC article. - Chm-1 gene-modified bone marrow mesenchymal stem cells maintain the chondrogenic phenotype of tissue-engineered cartilage.
Chen Z, Wei J, Zhu J, Liu W, Cui J, Li H, Chen F. Chen Z, et al. Stem Cell Res Ther. 2016 May 5;7(1):70. doi: 10.1186/s13287-016-0328-x. Stem Cell Res Ther. 2016. PMID: 27150539 Free PMC article. - Bioprinting of Chondrocyte Stem Cell Co-Cultures for Auricular Cartilage Regeneration.
Posniak S, Chung JHY, Liu X, Mukherjee P, Gambhir S, Khansari A, Wallace GG. Posniak S, et al. ACS Omega. 2022 Feb 11;7(7):5908-5920. doi: 10.1021/acsomega.1c06102. eCollection 2022 Feb 22. ACS Omega. 2022. PMID: 35224351 Free PMC article. - Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering.
Kang H, Zeng Y, Varghese S. Kang H, et al. Acta Biomater. 2018 Sep 15;78:365-377. doi: 10.1016/j.actbio.2018.07.039. Epub 2018 Jul 19. Acta Biomater. 2018. PMID: 30031911 Free PMC article. - Ca2+/Calmodulin-dependent Protein Kinase II Inhibitor KN-93 Enhances Chondrogenesis of Bone Marrow Mesenchymal Stem Cells and Delays Chondrogenic Hypertrophy.
Wuttisiriboon K, Tippayawat P, Daduang J, Limpaiboon T. Wuttisiriboon K, et al. In Vivo. 2023 Mar-Apr;37(2):667-678. doi: 10.21873/invivo.13127. In Vivo. 2023. PMID: 36881077 Free PMC article.
References
- Williams C.G. Kim T.K. Taboas A. Malik A. Manson P. Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9:679. - PubMed
- Friedenstein A.J. Piatetzky S., II Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381. - PubMed
- Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources