Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Aβ oligomers - PubMed (original) (raw)
doi: 10.1186/1750-1326-5-57.
Isabel Canto, Leonid Breydo, Suhail Rasool, Tamas Lukacsovich, Jessica Wu, Ricardo Albay 3rd, Anna Pensalfini, Stephen Yeung, Elizabeth Head, J Lawrence Marsh, Charles Glabe
Affiliations
- PMID: 21144050
- PMCID: PMC3019145
- DOI: 10.1186/1750-1326-5-57
Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Aβ oligomers
Rakez Kayed et al. Mol Neurodegener. 2010.
Abstract
Background: Age-related neurodegenerative diseases share a number of important pathological features, such as accumulation of misfolded proteins as amyloid oligomers and fibrils. Recent evidence suggests that soluble amyloid oligomers and not the insoluble amyloid fibrils may represent the primary pathological species of protein aggregates.
Results: We have produced several monoclonal antibodies that specifically recognize prefibrillar oligomers and do not recognize amyloid fibrils, monomer or natively folded proteins. Like the polyclonal antisera, the individual monoclonals recognize generic epitopes that do not depend on a specific linear amino acid sequence, but they display distinct preferences for different subsets of prefibrillar oligomers. Immunological analysis of a number of different prefibrillar Aβ oligomer preparations show that structural polymorphisms exist in Aβ prefibrillar oligomers that can be distinguished on the basis of their reactivity with monoclonal antibodies. Western blot analysis demonstrates that the conformers defined by the monoclonal antibodies have distinct size distributions, indicating that oligomer structure varies with size. The different conformational types of Aβ prefibrillar oligomers can serve as they serve as templates for monomer addition, indicating that they seed the conversion of Aβ monomer into more prefibrillar oligomers of the same type.
Conclusions: These results indicate that distinct structural variants or conformers of prefibrillar Aβ oligomers exist that are capable of seeding their own replication. These conformers may be analogous to different strains of prions.
Figures
Figure 1
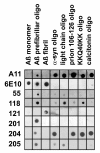
Dot blot analysis of monoclonal antibody specificity. Nitrocellulose strips containing 1 ug spots of Aβ40 monomer, PFOs and fibrils and prefibrillar oligomers prepared from α-synuclein, immunoglobulin light chain, prion 106-126 peptide, KK(Q40)KK peptide, and calcitonin were probed with the monoclonal antibodies and with A11 polyclonal and 6E10 control antibodies. A11 stains all prefibrillar oligomers samples, while 6E10 recognizes only samples containing Aβ. The individual monoclonal antibodies only recognize Aβ PFOs and not Aβ monomer or fibril samples, but display distinct preferences for other types of PFOs. Mab204 displays the broadest reactivity, recognizing all PFOs except for light chain, while Mab 201 is the most restrictive reacting with only Aβ PFOs.
Figure 2
Sequence comparison of monoclonal antibodies. Five monoclonal antibody mRNAs were cloned and sequenced. Alignment of heavy chain (A.) and light chain (B.) variable region amino acid sequences by the Clustal V method is shown. Regions of identical sequence are shown in red, while highly variable regions are shown in blue. Regions of increasing similarity are shown in colors of increasing wavelength.
Figure 3
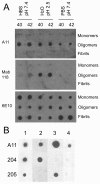
Monoclonal antibodies distinguish conformational variants of Aβ PFOs. A. Aβ40 (40) and Aβ42 (42) were incubated under three different conditions: HEPES buffered saline pH7.4, water pH 2.5 or PBS pH 7.4. Monomer samples were spotted at time zero, while prefibrillar oligomers and fibrils were spotted at 72 and 240 hours of incubation respectively. All of the oligomer samples stain with A11, but M118 only reacts with the Aβ samples prepared at pH 2.5. B. Four different samples of prefibrillar oligomers were prepared in water pH 2.5 and stained with A11, M204 and M205. All samples stain with A11 (top row). Sample 1 stains with both 204 and 205 antibodies while sample 2 stains with only M204, sample 3 stains only with M205 and sample 4 stains with neither monoclonal antibody.
Figure 4
Western blot analysis of monoclonal antibody PFO specificity. A. Two different Aβ40 PFOs samples (O1 and O2) and Aβ40 "monomer" (M) were prepared as described in Methods. None of the PFO specific monoclonal antibodies stain Aβ monomer, although the "monomer" sample is recognized by the 6E10 control antibody. Different Mabs stain distinct size distributions of Aβ PFOs. OC polyclonal sera stains high MW bands in oligomer preparation O1 and low MW bands in preparation O2. B. PFO specific monoclonal antibodies recognize soluble oligomers of Aβ42 and other types of amyloids. Soluble oligomers were prepared from Aβ, PrP 109-149 and α-synuclein as described in Methods. Aggregates were analyzed by Western blot with 6E10, A11, and monoclonal antibodies 55, 204, and 205. 6E10 only recognizes Aβ peptide. A11 detects oligomers of different sizes that are formed by Aβ and other types of amyloids. Monoclonal antibodies preferentially recognize different subsets of A11 positive oligomers.
Figure 5
Prefibrillar oligomers seed prefibrillar oligomer formation from monomer. A. The presence of a small amount of ladder like 10 - 56 kDa PFO seeds (prepared by dilution of NaOH stock solutions of Aβ40 into10 mM phosphate buffer pH 7.4), accelerates oligomer formation and gives rise to oligomers that stain with A11. No PFO immunoreactivity is observed in the non-seeded samples under these conditions. B. Mab 205 positive 35 - 45 kDa PFOs seed their own formation from Aβ40 monomer. C. Mab 205 35 - 45 kDa Aβ40 and Aβ42 PFOs seed the formation of A11 positive 35 - 45 kDa oligomers from Aβ42 monomers. Only the 35 - 45 kDa band is detected by A11, indicating that other sizes of oligomers are not formed. D. Seeding with PFOs suppresses the formation of fibrillar oligomers. In comparison, FOs spontaneously form in unseeded samples.
Figure 6
A11-like monoclonal antibodies do not stain amyloid plaques in human AD brain. Sections of frontal cortex (Broadman's area 11) were stained with Mabs 55, 18, 204 and 205 and 6E10 as a control. The bar indicates 200 μm.
Similar articles
- Fibril specific, conformation dependent antibodies recognize a generic epitope common to amyloid fibrils and fibrillar oligomers that is absent in prefibrillar oligomers.
Kayed R, Head E, Sarsoza F, Saing T, Cotman CW, Necula M, Margol L, Wu J, Breydo L, Thompson JL, Rasool S, Gurlo T, Butler P, Glabe CG. Kayed R, et al. Mol Neurodegener. 2007 Sep 26;2:18. doi: 10.1186/1750-1326-2-18. Mol Neurodegener. 2007. PMID: 17897471 Free PMC article. - Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity.
Ladiwala AR, Litt J, Kane RS, Aucoin DS, Smith SO, Ranjan S, Davis J, Van Nostrand WE, Tessier PM. Ladiwala AR, et al. J Biol Chem. 2012 Jul 13;287(29):24765-73. doi: 10.1074/jbc.M111.329763. Epub 2012 Apr 30. J Biol Chem. 2012. PMID: 22547072 Free PMC article. - Fibrillar oligomers nucleate the oligomerization of monomeric amyloid beta but do not seed fibril formation.
Wu JW, Breydo L, Isas JM, Lee J, Kuznetsov YG, Langen R, Glabe C. Wu JW, et al. J Biol Chem. 2010 Feb 26;285(9):6071-9. doi: 10.1074/jbc.M109.069542. Epub 2009 Dec 15. J Biol Chem. 2010. PMID: 20018889 Free PMC article. - Structural classification of toxic amyloid oligomers.
Glabe CG. Glabe CG. J Biol Chem. 2008 Oct 31;283(44):29639-43. doi: 10.1074/jbc.R800016200. Epub 2008 Aug 22. J Biol Chem. 2008. PMID: 18723507 Free PMC article. Review. - Amyloid oligomers: formation and toxicity of Abeta oligomers.
Sakono M, Zako T. Sakono M, et al. FEBS J. 2010 Mar;277(6):1348-58. doi: 10.1111/j.1742-4658.2010.07568.x. Epub 2010 Feb 9. FEBS J. 2010. PMID: 20148964 Review.
Cited by
- Conformation-dependent oligomers in cerebrospinal fluid of presymptomatic familial Alzheimer's disease mutation carriers.
Ringman JM, Tomic JL, Coppola G, Elashoff D, Gylys KH, Glabe CG. Ringman JM, et al. Dement Geriatr Cogn Dis Extra. 2012 Jan;2(1):652-7. doi: 10.1159/000345771. Epub 2012 Dec 15. Dement Geriatr Cogn Dis Extra. 2012. PMID: 23341831 Free PMC article. - Monoclonal antibodies against Aβ42 fibrils distinguish multiple aggregation state polymorphisms in vitro and in Alzheimer disease brain.
Hatami A, Albay R 3rd, Monjazeb S, Milton S, Glabe C. Hatami A, et al. J Biol Chem. 2014 Nov 14;289(46):32131-32143. doi: 10.1074/jbc.M114.594846. Epub 2014 Oct 3. J Biol Chem. 2014. PMID: 25281743 Free PMC article. - Evaluation of a DNA Aβ42 Vaccine in Aged NZW Rabbits: Antibody Kinetics and Immune Profile after Intradermal Immunization with Full-Length DNA Aβ42 Trimer.
Lambracht-Washington D, Fu M, Wight-Carter M, Riegel M, Rosenberg RN. Lambracht-Washington D, et al. J Alzheimers Dis. 2017;57(1):97-112. doi: 10.3233/JAD-160947. J Alzheimers Dis. 2017. PMID: 28222511 Free PMC article. - Natural Compound from Olive Oil Inhibits S100A9 Amyloid Formation and Cytotoxicity: Implications for Preventing Alzheimer's Disease.
Leri M, Chaudhary H, Iashchishyn IA, Pansieri J, Svedružić ŽM, Gómez Alcalde S, Musteikyte G, Smirnovas V, Stefani M, Bucciantini M, Morozova-Roche LA. Leri M, et al. ACS Chem Neurosci. 2021 Jun 2;12(11):1905-1918. doi: 10.1021/acschemneuro.0c00828. Epub 2021 May 12. ACS Chem Neurosci. 2021. PMID: 33979140 Free PMC article. - Vaccination with a non-human random sequence amyloid oligomer mimic results in improved cognitive function and reduced plaque deposition and micro hemorrhage in Tg2576 mice.
Rasool S, Albay R 3rd, Martinez-Coria H, Breydo L, Wu J, Milton S, Misra S, Tran A, Pensalfini A, Laferla F, Kayed R, Glabe CG. Rasool S, et al. Mol Neurodegener. 2012 Aug 6;7:37. doi: 10.1186/1750-1326-7-37. Mol Neurodegener. 2012. PMID: 22866920 Free PMC article.
References
- Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol. 1996;55(10):1023–1025. - PubMed
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Annals of Neurology. 1999;46(6):860–866. doi: 10.1002/1531-8249(199912)46:6<860::AID-ANA8>3.0.CO;2-M. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources