Signaling cell death from the endoplasmic reticulum stress response - PubMed (original) (raw)
Review
Signaling cell death from the endoplasmic reticulum stress response
Gordon C Shore et al. Curr Opin Cell Biol. 2011 Apr.
Abstract
Inability to meet protein folding demands within the endoplasmic reticulum (ER) activates the unfolded protein response (UPR), a signaling pathway with both adaptive and apoptotic outputs. While some secretory cell types have a remarkable ability to increase protein folding capacity, their upper limits can be reached when pathological conditions overwhelm the fidelity and/or output of the secretory pathway. Irremediable 'ER stress' induces apoptosis and contributes to cell loss in several common human diseases, including type 2 diabetes and neurodegeneration. Researchers have begun to elucidate the molecular switches that determine when ER stress is too great to repair and the signals that are then sent from the UPR to execute the cell.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
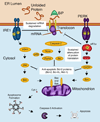
Figure 1. Connections from the UPR to the Mitochondrial Apoptotic Pathway
Under excessive ER stress, the ER transmembrane sensors IRE1α and PERK send signals through the BCL-2 family of proteins to activate the mitochondrial apoptotic pathway. In response to unfolded proteins, IRE1α oligomerizes and induces endonucleolytic decay of hundreds of ER-localized mRNAs, depleting ER protein folding components and leading to worsening ER stress. Phosphorylated IRE1α also recruits TNF receptor-associated factor 2 (TRAF2) and activates apoptosis signaling kinase 1 (ASK1) and its downstream target c-Jun NH2-terminal kinase (JNK). JNK then activates pro-apoptotic BIM and inhibits anti-apoptotic BCL-2. These conditions result in dimerization of PERK and activation of its kinase domain to phosphorylate eukaryotic translation initiation factor 2α (eIF2α), which causes selective translation of activating transcription factor-4 (ATF4). ATF4 upregulates expression of the CHOP/GADD153 transcription factor, which inhibits the gene encoding anti-apoptotic BCL-2 while inducing expression of pro-apoptotic BIM. ER stress also promotes p53-dependent transcriptional upregulation of Noxa and Puma, two additional pro-apoptotic BH3-only proteins. Furthermore, high levels of UPR signaling induce initiator caspase-2 to proteolytically cleave and activate pro-apoptotic BID upstream of the mitochondrion. In addition to antagonizing pro-survival BCL-2 members, cleaved BID, BIM and PUMA activate Bax and/or Bak. Hence, in response to excessive UPR signaling, the balance of BCL-2 family proteins shifts in the direction of apoptosis and leads to the oligomerization of BAX and BAK, two multi-domain pro-apoptotic BCL-2 family proteins that then drive the permeabilization of the outer mitochondrial membrane, apoptosome formation and activation of executioner caspases such as Caspase-3. Figure adapted with permission from the Journal of Cell Science [58].
Similar articles
- Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.
Elwenspoek MM, Thom H, Sheppard AL, Keeney E, O'Donnell R, Jackson J, Roadevin C, Dawson S, Lane D, Stubbs J, Everitt H, Watson JC, Hay AD, Gillett P, Robins G, Jones HE, Mallett S, Whiting PF. Elwenspoek MM, et al. Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article. - Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.
Manes TJ, DeGenova DT, Taylor BC, Patel JN. Manes TJ, et al. JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
Cited by
- Novel compound C150 inhibits pancreatic cancer through induction of ER stress and proteosome assembly.
Wang T, Chen P, Weir S, Baltezor M, Schoenen FJ, Chen Q. Wang T, et al. Front Oncol. 2022 Oct 5;12:870473. doi: 10.3389/fonc.2022.870473. eCollection 2022. Front Oncol. 2022. PMID: 36276125 Free PMC article. - Erratum: Proteostasis control by the unfolded protein response.
Hetz C, Chevet E, Oakes SA. Hetz C, et al. Nat Cell Biol. 2015 Aug;17(8):1088. doi: 10.1038/ncb3221. Nat Cell Biol. 2015. PMID: 26239530 Free PMC article. - Subamolide B Isolated from Medicinal Plant Cinnamomum subavenium Induces Cytotoxicity in Human Cutaneous Squamous Cell Carcinoma Cells through Mitochondrial and CHOP-Dependent Cell Death Pathways.
Yang SY, Wang HM, Wu TW, Chen YJ, Shieh JJ, Lin JH, Ho TF, Luo RJ, Chen CY, Chang CC. Yang SY, et al. Evid Based Complement Alternat Med. 2013;2013:630415. doi: 10.1155/2013/630415. Epub 2013 Mar 13. Evid Based Complement Alternat Med. 2013. PMID: 23573140 Free PMC article. - Acute hypoxia induces apoptosis of pancreatic β-cell by activation of the unfolded protein response and upregulation of CHOP.
Zheng X, Zheng X, Wang X, Ma Z, Gupta Sunkari V, Botusan I, Takeda T, Björklund A, Inoue M, Catrina SB, Brismar K, Poellinger L, Pereira TS. Zheng X, et al. Cell Death Dis. 2012 Jun 14;3(6):e322. doi: 10.1038/cddis.2012.66. Cell Death Dis. 2012. PMID: 22695615 Free PMC article. - CNS Redox Homeostasis and Dysfunction in Neurodegenerative Diseases.
Goldsteins G, Hakosalo V, Jaronen M, Keuters MH, Lehtonen Š, Koistinaho J. Goldsteins G, et al. Antioxidants (Basel). 2022 Feb 16;11(2):405. doi: 10.3390/antiox11020405. Antioxidants (Basel). 2022. PMID: 35204286 Free PMC article. Review.
References
- Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. ••Report that Chop signals ER stress-induced apoptosis in a diabetes model.
- Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. - PubMed
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 CA136577/CA/NCI NIH HHS/United States
- CAPMC/ CIHR/Canada
- R01 CA136577-01A1/CA/NCI NIH HHS/United States
- DP2 OD001925/OD/NIH HHS/United States
- K08 AI054650-01/AI/NIAID NIH HHS/United States
- K08 AI054650/AI/NIAID NIH HHS/United States
- R01 DK080955/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources