Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases - PubMed (original) (raw)
Review
Chemical and/or biological therapeutic strategies to ameliorate protein misfolding diseases
Derrick Sek Tong Ong et al. Curr Opin Cell Biol. 2011 Apr.
Abstract
Inheriting a mutant misfolding-prone protein that cannot be efficiently folded in a given cell type(s) results in a spectrum of human loss-of-function misfolding diseases. The inability of the biological protein maturation pathways to adapt to a specific misfolding-prone protein also contributes to pathology. Chemical and biological therapeutic strategies are presented that restore protein homeostasis, or proteostasis, either by enhancing the biological capacity of the proteostasis network or through small molecule stabilization of a specific misfolding-prone protein. Herein, we review the recent literature on therapeutic strategies to ameliorate protein misfolding diseases that function through either of these mechanisms, or a combination thereof, and provide our perspective on the promise of alleviating protein misfolding diseases by taking advantage of proteostasis adaptation.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
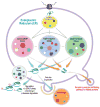
Figure 1. The Proteostasis Network
Schematic of the endoplasmic reticulum (ER) proteostasis network in mammalian cells depicting the components and the connections in simplified format. A holdase chaperone delivers the nascent chain to the Hsp-70-40-nucleotide exchange factor pathway and/or the Hsp90-cochaperone pathway and/or the Calnexin-calreticulin pathway which can lead to folding and vesicular trafficking or degradation mediated by ER-associated degradation (proteasome) and/or by autophagy.
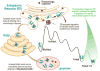
Figure 2. Pharmacologic Chaperoning
A small molecule pharmacologic chaperone binds to the folded ensemble of a specific mutant misfolding-prone lysosomal storage disease-associated enzyme in the endoplasmic reticulum (ER) and stabilizes the mutant enzyme. This increases the fraction of folded enzyme that can bind to the trafficking receptor and be trafficked to the lysosome to degrade its substrate.
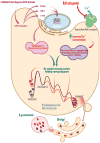
Figure 3. Adapting the Proteostasis Network
Small molecule proteostasis regulators can induce the unfolded protein response (UPR) transcriptional program leading to the coordinated upregulation of endoplasmic reticulum (ER) proteostasis network capacity. The chaperones, co-chaperones and folding enzymes can resculpt the folding free energy diagram of mutant enzymes, “pushing” more protein toward the native state by lowering the energy of intermediate and transition states and minimizing misfolding. Small molecule proteostasis regulators can also function by post-translation regulation mechanisms. One example are the Ryanodine receptor antagonists that increase the ER Ca2+ concentration leading to more Ca2+ binding by the chaperones, including calnexin and calreticulin, increasing their activity and ability to resculpt the folding free energy diagram of mutant lysosomal enzymes by “pushing” more protein toward the native state at the expense of misfolding.
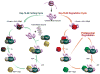
Figure 4. Competition between Protein Folding and Degradation is a Central Feature of Proteostasis Network Function
Depicted is an Hsp-70-40-nucleotide exchange proteostasis pathway that is intentionally generic (not organelle specific), illustrating how the competition between folding and degradation of a foldable protein is affected. As can be discerned from this representative pathway, the concentrations and activity of many components (many not shown) influence the ratio of folding to degradation for a particular client protein.
Similar articles
- Underlying mechanisms and chemical/biochemical therapeutic approaches to ameliorate protein misfolding neurodegenerative diseases.
Hekmatimoghaddam S, Zare-Khormizi MR, Pourrajab F. Hekmatimoghaddam S, et al. Biofactors. 2017 Nov;43(6):737-759. doi: 10.1002/biof.1264. Epub 2016 Feb 22. Biofactors. 2017. PMID: 26899445 Review. - Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis.
Lindquist SL, Kelly JW. Lindquist SL, et al. Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a004507. doi: 10.1101/cshperspect.a004507. Cold Spring Harb Perspect Biol. 2011. PMID: 21900404 Free PMC article. Review. - Pharmacoperones as Novel Therapeutics for Diverse Protein Conformational Diseases.
Tao YX, Conn PM. Tao YX, et al. Physiol Rev. 2018 Apr 1;98(2):697-725. doi: 10.1152/physrev.00029.2016. Physiol Rev. 2018. PMID: 29442594 Free PMC article. Review. - Proteostasis: bad news and good news from the endoplasmic reticulum.
Noack J, Brambilla Pisoni G, Molinari M. Noack J, et al. Swiss Med Wkly. 2014 Aug 21;144:w14001. doi: 10.4414/smw.2014.14001. eCollection 2014. Swiss Med Wkly. 2014. PMID: 25144910 Review. - Pharmacologic Approaches for Adapting Proteostasis in the Secretory Pathway to Ameliorate Protein Conformational Diseases.
Kelly JW. Kelly JW. Cold Spring Harb Perspect Biol. 2020 May 1;12(5):a034108. doi: 10.1101/cshperspect.a034108. Cold Spring Harb Perspect Biol. 2020. PMID: 31088828 Free PMC article. Review.
Cited by
- A chaperone trap contributes to the onset of cystic fibrosis.
Coppinger JA, Hutt DM, Razvi A, Koulov AV, Pankow S, Yates JR 3rd, Balch WE. Coppinger JA, et al. PLoS One. 2012;7(5):e37682. doi: 10.1371/journal.pone.0037682. Epub 2012 May 31. PLoS One. 2012. PMID: 22701530 Free PMC article. - Histone deacetylase inhibitor (HDACi) suberoylanilide hydroxamic acid (SAHA)-mediated correction of α1-antitrypsin deficiency.
Bouchecareilh M, Hutt DM, Szajner P, Flotte TR, Balch WE. Bouchecareilh M, et al. J Biol Chem. 2012 Nov 2;287(45):38265-78. doi: 10.1074/jbc.M112.404707. Epub 2012 Sep 20. J Biol Chem. 2012. PMID: 22995909 Free PMC article. - Podocyte Injury Associated with Mutant α-Actinin-4.
Cybulsky AV, Kennedy CR. Cybulsky AV, et al. J Signal Transduct. 2011;2011:563128. doi: 10.1155/2011/563128. Epub 2011 Jul 26. J Signal Transduct. 2011. PMID: 21808733 Free PMC article. - Caught in the act: covalent cross-linking captures activator-coactivator interactions in vivo.
Krishnamurthy M, Dugan A, Nwokoye A, Fung YH, Lancia JK, Majmudar CY, Mapp AK. Krishnamurthy M, et al. ACS Chem Biol. 2011 Dec 16;6(12):1321-6. doi: 10.1021/cb200308e. Epub 2011 Nov 14. ACS Chem Biol. 2011. PMID: 21977905 Free PMC article. - Carnitine is a pharmacological allosteric chaperone of the human lysosomal _α_-glucosidase.
Iacono R, Minopoli N, Ferrara MC, Tarallo A, Damiano C, Porto C, Strollo S, Roig-Zamboni V, Peluso G, Sulzenbacher G, Cobucci-Ponzano B, Parenti G, Moracci M. Iacono R, et al. J Enzyme Inhib Med Chem. 2021 Dec;36(1):2068-2079. doi: 10.1080/14756366.2021.1975694. J Enzyme Inhib Med Chem. 2021. PMID: 34565280 Free PMC article.
References
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. Review describing the proteostasis network, how deficiencies in proteostasis results in various human diseases, and the utility of proteostasis regulators to manipulate the concentration, conformation, quaternary structure, and/or location of the protein(s) to ameliorate these diseases. - PubMed
- Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency. Annu Rev Biochem. 2009;78:23.21–33. - PubMed
- Deuerling E, Bukau B. Chaperone-assisted folding of newly synthesized proteins in the cytosol. Crit Rev Biochem Mol Biol. 2004;39:261–277. - PubMed
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources