Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action - PubMed (original) (raw)
Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action
Jun-Li Cao et al. J Neurosci. 2010.
Abstract
We previously reported that the activity of mesolimbic dopamine neurons of the ventral tegmental area (VTA) is a key determinant of behavioral susceptibility vs resilience to chronic social defeat stress. However, this was based solely on ex vivo measurements, and the in vivo firing properties of VTA dopamine neurons in susceptible and resilient mice, as well as the effects of antidepressant treatments, remain completely unknown. Here, we show that chronic (10 d) social defeat stress significantly increased the in vivo spontaneous firing rates and bursting events in susceptible mice but not in the resilient subgroup. Both the firing rates and bursting events were significantly negatively correlated with social avoidance behavior, a key behavioral abnormality induced by chronic social defeat stress. Moreover, the increased firing rates, bursting events, and avoidance behavior in susceptible mice were completely reversed by chronic (2 week), but not acute (single dose), treatments with the antidepressant medication fluoxetine (20 mg/kg). Chronic social defeat stress increased hyperpolarization-activated cation current (I(h)) in VTA dopamine neurons, an effect that was also normalized by chronic treatment with fluoxetine. As well, local infusion of I(h) inhibitors ZD7288 (0.1 μg) or DK-AH 269 (0.6 μg) into the VTA exerted antidepressant-like behavioral effects. Together, these data suggest that the firing patterns of mesolimbic dopamine neurons in vivo mediate an individual's responses to chronic stress and antidepressant action.
Figures
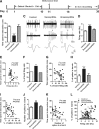
Figure 1.
Chronic social defeat stress increases the firing rates and bursting events in VTA DA neurons in susceptible, not resilient (unsusceptible), mice. A, Timeline of chronic social defeat, behavioral testing, and in vivo recording protocols. B, The time spent in the interaction zone during a social interaction test (***p < 0.0001 vs control or unsusceptible; 8–20 mice/group). C, Sample traces, bursting events, and spikes for in vivo recordings from VTA DA neurons from control, susceptible, and unsusceptible mice. D, Overall VTA firing rates in control, susceptible, and unsusceptible mice (***p < 0.0001 vs control or unsusceptible mice, n = 58–72 cells, 15 mice/group). E, Average VTA firing rate for each mouse is significantly negatively correlated with the time spent in interaction zone measured on day 11 (p < 0.05, n = 35 mice). F, H, J, Burst-firing events of VTA DA neurons, including percentage of burst-firing cells, percentage of spikes in bursts, and number of spikes in a burst, were increased in susceptible, not unsusceptible, mice (**p < 0.01, ***p < 0.001 vs control or unsusceptible mice; n = 23–49 cells, 8–15 mice/group). G, I, K, The average changes of these bursting events for each animal also inversely correlated with its social interaction time (p < 0.05; n = 35 mice). L, Scatter plot of the bursting activity and firing rates of all neurons recorded from control, susceptible, and unsusceptible mice. Note the large number of VTA DA neurons in susceptible mice with high firing rate and more bursting activity. Firing rates are significantly positively correlated with percentage of spikes in bursts in all three groups, suggesting the increased firing rates were associated with the increase of bursting events (control: r 2 = 0.127, n = 65 cells/15 mice, p < 0.001; susceptible: r 2 = 0.0952, n = 72 cells/12 mice, p < 0.001; unsusceptible: r 2 = 0.0758, n = 58 cells/8 mice, p < 0.05).
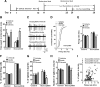
Figure 2.
Chronic treatment with fluoxetine normalizes stress-induced maladaptations of VTA DA neurons in susceptible mice. A, Timeline of chronic social defeat, behavioral testing, treatment with fluoxetine, and in vivo recording protocols. B, Chronic, not acute, administration of fluoxetine normalized defeat-induced avoidance behavior in susceptible mice (**p < 0.01 vs susceptible-vehicle mice, 5–7 mice/group). **_C_**, Sample traces for _in vivo_ recordings from VTA DA neurons from susceptible mice with acute or chronic treatment with vehicle or fluoxetine. **_D_**, Cumulative probability distribution plots of VTA firing rates in susceptible mice after acute or chronic treatment with vehicle or fluoxetine. **_E_**, Acute or chronic treatment with vehicle or fluoxetine did not change the firing rates of VTA DA neurons in control mice (_p_ > 0.5, n = 46–48 cells, 5 mice/group), and chronic, not acute, administration of fluoxetine normalized the increased firing rates of VTA DA neurons in susceptible mice (***p < 0.001 vs susceptible-vehicle or susceptible-acute mice; _n_ = 43–64 cells, 5–6 mice/group). **_F–H_**, Acute or chronic treatment with vehicle or fluoxetine did not change burst events of VTA DA neurons in control mice (_p_ > 0.5, n = 20–21 cells, 5 mice/group), however, chronic, not acute, administration of fluoxetine reversed the increased burst events of VTA DA neurons in susceptible mice (*p < 0.05, **p < 0.01 vs susceptible-vehicle or susceptible-acute mice, n = 22–45 cells, 5–6 mice/group). I, Scatter plot of percentage of spikes in bursts and firing rates of all neurons recorded from susceptible-vehicle (n = 61), susceptible-acute (n = 50), and susceptible-chronic (n = 43) mice. Note the small number of VTA DA neurons with high firing rates and more bursting activity in susceptible mice after chronic treatment with fluoxetine.
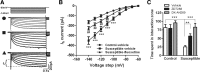
Figure 3.
Ih channels in VTA DA neurons were involved in defeat-induced avoidance behavior and action of fluoxetine. A, Ih currents were elicited by repetitive 800 ms pulses with 10 mV increments from a holding potential of −60 to −140 mV. Sample traces recorded from control-vehicle, susceptible-vehicle, and susceptible-fluoxetine mice. B, Chronic social defeat increased Ih currents in VTA DA neurons, which was almost completely reversed by chronic treatment with fluoxetine (***p < 0.0001 vs control-vehicle mice, ## p < 0.01 vs susceptible-fluoxetine mice, n = 35–66 cells, 10–12 mice/group). C, Infusion of Ih channel inhibitor ZD7288 or DK-AH 269 into VTA significantly reversed the stress-induced social avoidance (**p < 0.01, ***p < 0.001; n = 6–11 mice).
Similar articles
- Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors.
Wook Koo J, Labonté B, Engmann O, Calipari ES, Juarez B, Lorsch Z, Walsh JJ, Friedman AK, Yorgason JT, Han MH, Nestler EJ. Wook Koo J, et al. Biol Psychiatry. 2016 Sep 15;80(6):469-478. doi: 10.1016/j.biopsych.2015.12.009. Epub 2015 Dec 15. Biol Psychiatry. 2016. PMID: 26858215 Free PMC article. - Prostaglandin E2-mediated attenuation of mesocortical dopaminergic pathway is critical for susceptibility to repeated social defeat stress in mice.
Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E, Deguchi Y, Breyer RM, Breyer MD, Narumiya S. Tanaka K, et al. J Neurosci. 2012 Mar 21;32(12):4319-29. doi: 10.1523/JNEUROSCI.5952-11.2012. J Neurosci. 2012. PMID: 22442093 Free PMC article. - Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Chaudhury D, et al. Nature. 2013 Jan 24;493(7433):532-6. doi: 10.1038/nature11713. Epub 2012 Dec 12. Nature. 2013. PMID: 23235832 Free PMC article. - Role of Mesolimbic Brain-Derived Neurotrophic Factor in Depression.
Koo JW, Chaudhury D, Han MH, Nestler EJ. Koo JW, et al. Biol Psychiatry. 2019 Nov 15;86(10):738-748. doi: 10.1016/j.biopsych.2019.05.020. Epub 2019 Jun 4. Biol Psychiatry. 2019. PMID: 31327473 Free PMC article. Review. - Neurotrophins in the ventral tegmental area: Role in social stress, mood disorders and drug abuse.
Nikulina EM, Johnston CE, Wang J, Hammer RP Jr. Nikulina EM, et al. Neuroscience. 2014 Dec 12;282:122-38. doi: 10.1016/j.neuroscience.2014.05.028. Epub 2014 May 27. Neuroscience. 2014. PMID: 24875178 Free PMC article. Review.
Cited by
- Mouse Testing Methods in Psychoneuroimmunology: Measuring Behavioral Responses.
Krauklis SA, Towers AE, York JM, Baynard T, Gainey SJ, Freund GG, Steelman AJ. Krauklis SA, et al. Methods Mol Biol. 2025;2868:163-203. doi: 10.1007/978-1-0716-4200-9_10. Methods Mol Biol. 2025. PMID: 39546231 - Alteration of hyperpolarization-activated cation current-mediated metaplasticity contributes to electroconvulsive shock-induced learning and memory impairment in depressed rats.
Ren L, Yu J, Chen H, Luo J, Lv F, Min S. Ren L, et al. Front Psychiatry. 2024 Jun 7;15:1365119. doi: 10.3389/fpsyt.2024.1365119. eCollection 2024. Front Psychiatry. 2024. PMID: 38911706 Free PMC article. - Midbrain glutamatergic circuit mechanism of resilience to socially transferred allodynia in male mice.
Han Y, Ai L, Song L, Zhou Y, Chen D, Sha S, Ji R, Li Q, Bu Q, Pan X, Zhai X, Cui M, Duan J, Yang J, Chaudhury D, Hu A, Liu H, Han MH, Cao JL, Zhang H. Han Y, et al. Nat Commun. 2024 Jun 10;15(1):4947. doi: 10.1038/s41467-024-49340-8. Nat Commun. 2024. PMID: 38858350 Free PMC article. - Optogenetic behavioral studies in depression research: A systematic review.
Spreen A, Alkhoury D, Walter H, Müller S. Spreen A, et al. iScience. 2024 Apr 18;27(5):109776. doi: 10.1016/j.isci.2024.109776. eCollection 2024 May 17. iScience. 2024. PMID: 38726370 Free PMC article. - Exploring causal mechanisms of psychosis risk.
Oliver D, Chesney E, Cullen AE, Davies C, Englund A, Gifford G, Kerins S, Lalousis PA, Logeswaran Y, Merritt K, Zahid U, Crossley NA, McCutcheon RA, McGuire P, Fusar-Poli P. Oliver D, et al. Neurosci Biobehav Rev. 2024 Jul;162:105699. doi: 10.1016/j.neubiorev.2024.105699. Epub 2024 May 6. Neurosci Biobehav Rev. 2024. PMID: 38710421 Free PMC article. Review.
References
- Anstrom KK, Woodward DJ. Restraint increases depaminergic bursting firing in awake rats. Neuropsychopharmacology. 2005;30:1832–1840. - PubMed
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. - PubMed
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. - PubMed
MeSH terms
Substances
Grants and funding
- K01 DA017750/DA/NIDA NIH HHS/United States
- R01 MH051399/MH/NIMH NIH HHS/United States
- R01 MH051399-12/MH/NIMH NIH HHS/United States
- R01 MH092306/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials