Mixed-affinity binding in humans with 18-kDa translocator protein ligands - PubMed (original) (raw)
Mixed-affinity binding in humans with 18-kDa translocator protein ligands
David R J Owen et al. J Nucl Med. 2011 Jan.
Abstract
11C-PBR28 PET can detect the 18-kDa translocator protein (TSPO) expressed within macrophages. However, quantitative evaluation of the signal in brain tissue from donors with multiple sclerosis (MS) shows that PBR28 binds the TSPO with high affinity (binding affinity [Ki], ∼4 nM), low affinity (Ki, ∼200 nM), or mixed affinity (2 sites with Ki, ∼4 nM and ∼300 nM). Our study tested whether similar binding behavior could be detected in brain tissue from donors with no history of neurologic disease, with TSPO-binding PET ligands other than 11C-PBR28, for TSPO present in peripheral blood, and with human brain PET data acquired in vivo with 11C-PBR28.
Methods: The affinity of TSPO ligands was measured in the human brain postmortem from donors with a history of MS (n=13), donors without any history of neurologic disease (n=20), and in platelets from healthy volunteers (n=13). Binding potential estimates from thirty-five 11C-PBR28 PET scans from an independent sample of healthy volunteers were analyzed using a gaussian mixture model.
Results: Three binding affinity patterns were found in brains from subjects without neurologic disease in similar proportions to those reported previously from studies of MS brains. TSPO ligands showed substantial differences in affinity between subjects classified as high-affinity binders (HABs) and low-affinity binders (LABs). Differences in affinity between HABs and LABs are approximately 50-fold with PBR28, approximately 17-fold with PBR06, and approximately 4-fold with DAA1106, DPA713, and PBR111. Where differences in affinity between HABs and LABs were low (∼4-fold), distinct affinities were not resolvable in binding curves for mixed-affinity binders (MABs), which appeared to express 1 class of sites with an affinity approximately equal to the mean of those for HABs and LABs. Mixed-affinity binding was detected in platelets from an independent sample (HAB, 69%; MAB, 31%), although LABs were not detected. Analysis of 11C-PBR28 PET data was not inconsistent with the existence of distinct subpopulations of HABs, MABs, and LABs.
Conclusion: With the exception of 11C-PK11195, all TSPO PET ligands in current clinical application recognize HABs, LABs, and MABs in brain tissue in vitro. Knowledge of subjects' binding patterns will be required to accurately quantify TSPO expression in vivo using PET.
Figures
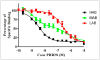
FIGURE 1
Competition assay with 3H-PK11195 and unlabeled PBR28, using tissue from donors with no history of neurologic disease. Each data point represents mean value of all subjects, and error bars represent SEM. Conc = concentration.
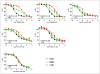
FIGURE 2
Competition assays with 3H-PK11195 and unlabeled TSPO ligand using brain tissue previously characterized as HAB, LAB, or MAB by PBR28 assays. Each data point represents mean value of at least 4 subjects, and error bars represent SEM. (A) Phenoxyphenyl acetamide derivatives. (B) Bicyclic linker derivatives. (C) Phenyl–isoquinolinecarboxamide derivatives. Conc = concentration; SB = specific binding.
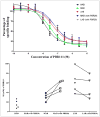
FIGURE 3
Competition assays with 3H-PK11195 and unlabeled PBR111 in presence or absence of PBR28 (50 nM).
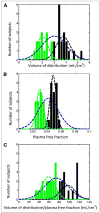
FIGURE 4
11C- PBR28 PET data from 32 healthy volunteers expressed as histogram with mixture model analysis showing 2 distributions for each measurement. (A) Volume of distribution. (B) Plasma free fraction. (C) Volume of distribution/plasma free fraction. Blue curves denote single-component solutions, and green and black curves denote 2-component solutions. For 2-component solutions, whereas different individuals are within each gaussian group (e.g., green) for 3 parameters, there was good agreement between A and B (74%) and A and C (66%).
FIGURE 5
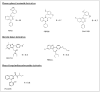
TSPO ligands displayed in structural classes.
Similar articles
- Quantification of the specific translocator protein signal of 18F-PBR111 in healthy humans: a genetic polymorphism effect on in vivo binding.
Guo Q, Colasanti A, Owen DR, Onega M, Kamalakaran A, Bennacef I, Matthews PM, Rabiner EA, Turkheimer FE, Gunn RN. Guo Q, et al. J Nucl Med. 2013 Nov;54(11):1915-23. doi: 10.2967/jnumed.113.121020. Epub 2013 Sep 26. J Nucl Med. 2013. PMID: 24071511 - Two binding sites for [3H]PBR28 in human brain: implications for TSPO PET imaging of neuroinflammation.
Owen DR, Howell OW, Tang SP, Wells LA, Bennacef I, Bergstrom M, Gunn RN, Rabiner EA, Wilkins MR, Reynolds R, Matthews PM, Parker CA. Owen DR, et al. J Cereb Blood Flow Metab. 2010 Sep;30(9):1608-18. doi: 10.1038/jcbfm.2010.63. Epub 2010 Apr 28. J Cereb Blood Flow Metab. 2010. PMID: 20424634 Free PMC article. - 11C-ER176, a Radioligand for 18-kDa Translocator Protein, Has Adequate Sensitivity to Robustly Image All Three Affinity Genotypes in Human Brain.
Ikawa M, Lohith TG, Shrestha S, Telu S, Zoghbi SS, Castellano S, Taliani S, Da Settimo F, Fujita M, Pike VW, Innis RB; Biomarkers Consortium Radioligand Project Team. Ikawa M, et al. J Nucl Med. 2017 Feb;58(2):320-325. doi: 10.2967/jnumed.116.178996. Epub 2016 Nov 17. J Nucl Med. 2017. PMID: 27856631 Free PMC article. - Have (R)-[11C]PK11195 challengers fulfilled the promise? A scoping review of clinical TSPO PET studies.
Chauveau F, Becker G, Boutin H. Chauveau F, et al. Eur J Nucl Med Mol Imaging. 2021 Dec;49(1):201-220. doi: 10.1007/s00259-021-05425-w. Epub 2021 Aug 13. Eur J Nucl Med Mol Imaging. 2021. PMID: 34387719 Free PMC article. Review. - Imaging brain microglial activation using positron emission tomography and translocator protein-specific radioligands.
Owen DR, Matthews PM. Owen DR, et al. Int Rev Neurobiol. 2011;101:19-39. doi: 10.1016/B978-0-12-387718-5.00002-X. Int Rev Neurobiol. 2011. PMID: 22050847 Review.
Cited by
- Mapping neuroinflammation in frontotemporal dementia with molecular PET imaging.
Zhang J. Zhang J. J Neuroinflammation. 2015 May 29;12:108. doi: 10.1186/s12974-015-0236-5. J Neuroinflammation. 2015. PMID: 26022249 Free PMC article. Review. - PET imaging of neuroinflammation: any credible alternatives to TSPO yet?
Chauveau F, Winkeler A, Chalon S, Boutin H, Becker G. Chauveau F, et al. Mol Psychiatry. 2025 Jan;30(1):213-228. doi: 10.1038/s41380-024-02656-9. Epub 2024 Jul 13. Mol Psychiatry. 2025. PMID: 38997465 Review. - Enhancing predictability of IDH mutation status in glioma patients at initial diagnosis: a comparative analysis of radiomics from MRI, [18F]FET PET, and TSPO PET.
Kaiser L, Quach S, Zounek AJ, Wiestler B, Zatcepin A, Holzgreve A, Bollenbacher A, Bartos LM, Ruf VC, Böning G, Thon N, Herms J, Riemenschneider MJ, Stöcklein S, Brendel M, Rupprecht R, Tonn JC, Bartenstein P, von Baumgarten L, Ziegler S, Albert NL. Kaiser L, et al. Eur J Nucl Med Mol Imaging. 2024 Jul;51(8):2371-2381. doi: 10.1007/s00259-024-06654-5. Epub 2024 Feb 24. Eur J Nucl Med Mol Imaging. 2024. PMID: 38396261 Free PMC article. - Association of Neurofilament Light Chain, [18F]PI-2620 Tau-PET, TSPO-PET, and Clinical Progression in Patients With β-Amyloid-Negative CBS.
Palleis C, Franzmeier N, Weidinger E, Bernhardt AM, Katzdobler S, Wall S, Ferschmann C, Harris S, Schmitt J, Schuster S, Gnörich J, Finze A, Biechele G, Lindner S, Albert NL, Bartenstein P, Sabri O, Barthel H, Rupprecht R, Nuscher B, Stephens AW, Rauchmann BS, Perneczky R, Haass C, Brendel M, Levin J, Höglinger GU. Palleis C, et al. Neurology. 2024 Jan 9;102(1):e207901. doi: 10.1212/WNL.0000000000207901. Epub 2023 Dec 14. Neurology. 2024. PMID: 38165362 Free PMC article. - Structural Integrity of the A147T Polymorph of Mammalian TSPO.
Jaremko M, Jaremko Ł, Giller K, Becker S, Zweckstetter M. Jaremko M, et al. Chembiochem. 2015 Jul 6;16(10):1483-9. doi: 10.1002/cbic.201500217. Epub 2015 Jun 10. Chembiochem. 2015. PMID: 25974690 Free PMC article.
References
- Banati RB, Newcombe J, Gunn RN, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123:2321–2337. - PubMed
- Yasuno F, Ota M, Kosaka J, et al. Increased binding of peripheral benzodiazepine receptor in Alzheimer’s disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry. 2008;64:835–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- WT_/Wellcome Trust/United Kingdom
- G0900897/MRC_/Medical Research Council/United Kingdom
- ZIA MH002852/ImNIH/Intramural NIH HHS/United States
- ZIA MH002852-06/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources