Subtypes of medulloblastoma have distinct developmental origins - PubMed (original) (raw)
. 2010 Dec 23;468(7327):1095-9.
doi: 10.1038/nature09587. Epub 2010 Dec 8.
Yiai Tong, Giles Robinson, Margaret C Thompson, D Spencer Currle, Christopher Eden, Tanya A Kranenburg, Twala Hogg, Helen Poppleton, Julie Martin, David Finkelstein, Stanley Pounds, Aaron Weiss, Zoltan Patay, Matthew Scoggins, Robert Ogg, Yanxin Pei, Zeng-Jie Yang, Sonja Brun, Youngsoo Lee, Frederique Zindy, Janet C Lindsey, Makoto M Taketo, Frederick A Boop, Robert A Sanford, Amar Gajjar, Steven C Clifford, Martine F Roussel, Peter J McKinnon, David H Gutmann, David W Ellison, Robert Wechsler-Reya, Richard J Gilbertson
Affiliations
- PMID: 21150899
- PMCID: PMC3059767
- DOI: 10.1038/nature09587
Subtypes of medulloblastoma have distinct developmental origins
Paul Gibson et al. Nature. 2010.
Abstract
Medulloblastoma encompasses a collection of clinically and molecularly diverse tumour subtypes that together comprise the most common malignant childhood brain tumour. These tumours are thought to arise within the cerebellum, with approximately 25% originating from granule neuron precursor cells (GNPCs) after aberrant activation of the Sonic Hedgehog pathway (hereafter, SHH subtype). The pathological processes that drive heterogeneity among the other medulloblastoma subtypes are not known, hindering the development of much needed new therapies. Here we provide evidence that a discrete subtype of medulloblastoma that contains activating mutations in the WNT pathway effector CTNNB1 (hereafter, WNT subtype) arises outside the cerebellum from cells of the dorsal brainstem. We found that genes marking human WNT-subtype medulloblastomas are more frequently expressed in the lower rhombic lip (LRL) and embryonic dorsal brainstem than in the upper rhombic lip (URL) and developing cerebellum. Magnetic resonance imaging (MRI) and intra-operative reports showed that human WNT-subtype tumours infiltrate the dorsal brainstem, whereas SHH-subtype tumours are located within the cerebellar hemispheres. Activating mutations in Ctnnb1 had little impact on progenitor cell populations in the cerebellum, but caused the abnormal accumulation of cells on the embryonic dorsal brainstem which included aberrantly proliferating Zic1(+) precursor cells. These lesions persisted in all mutant adult mice; moreover, in 15% of cases in which Tp53 was concurrently deleted, they progressed to form medulloblastomas that recapitulated the anatomy and gene expression profiles of human WNT-subtype medulloblastoma. We provide the first evidence, to our knowledge, that subtypes of medulloblastoma have distinct cellular origins. Our data provide an explanation for the marked molecular and clinical differences between SHH- and WNT-subtype medulloblastomas and have profound implications for future research and treatment of this important childhood cancer.
Conflict of interest statement
Competing interests.
None.
Figures
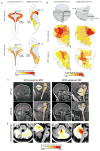
Figure 1. WNT and SHH-subtypes of medulloblastoma are anatomically distinct
(a) Expression distribution in (a) E11.5 and (b) E15.5 mouse hindbrain of orthologs that distinguish human WNT and SHH-subtype medulloblastoma (Supplemental Dataset 1). Cartoons in (b) denote the position of rhombomeres relative to the cerebellum and brainstem. (c) Top=pre and bottom=post-operative MRI scans of exemplary SHH and WNT-subtype medulloblastomas. Right panels show close up views of left. Brainstem (BSt), post-operative tumor cavity (cvt.). (d) Frequency and site of post-operative surgical cavities of SHH (n=6) and WNT (n=6)-subtype medulloblastomas. Axial (left) and sagittal (right) views are shown.
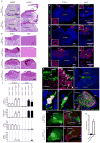
Figure 2. Mutant-Ctnnb1 causes aberrant accumulation of LRL cells
(a) Low (scale=180 μm) and (b) high (scale=50 μm) power views of LRL/dorsal brainstem in Ctnnb1 mutant and wild-type E16.5 embryos. (b) Includes the corresponding adult brainstem region. (c) Volume and indicated immunoreactivity differences between _Ctnnb1-_mutant and wild-type LRL (n≥3 mice per group, bars=mean ±S.D). Immunofluorescence of Olig3 (d), Pax6 (e), Zic1 (f) in _Ctnnb1-_mutant E16.5 LRL (left) and aberrant adult dorsal brainstem masses (right, scale=180 μm). Inset=high-power views of ‘*’ (scale=5 μm). (g) Postmitotic MF precursor neurons (Zic1+/Ki67-) exit the proliferating E16.5 control LRL. (h) _Ctnnb1-_mutant LRL contains aberrant proliferating Zic1+ precursors (arrows, scale=50 μm). (i) GFP-electroporated wild-type LRL marks Olig3+ cells (j) and migrating precursors (arrows in i) that include Zic1+ MF neurons (k) that form the PGN (l). GFP-fluorescence of whole (m,o) and sectioned (n,p) _Ctnnb1-_mutant and wild-type P0 hindbrains electroporated at E12.5. (q) Mean ± SD of LRL:PGN GFP-fluorescence in whole hindbrains of three BlBp-Cre ; Ctnnb1+/+ and five Blbp-Cre ; Ctnnb1+/lox(Ex3) mice (graphs, *≤0.05, **≤0.005, Exact Mann-Whitney P).
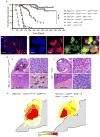
Figure 3. Mutant-Ctnnb1 and SHH-subtype mouse medulloblastomas are anatomically distinct
(a) Tumor free survival of SHH-subtype medulloblastoma mouse models (_Nes_-Cre+/−; Lig4flx/flx ; Tp53_−/_−, _Nes_-Cre+/−; Xrcc2flx/flx ; Tp53_−/_−, Ptch1+/−; Ink4c_−/_−, Ptch1+/−; Tp53_−/_− data from Refs.14,27,28) and _Ctnnb1-_mutant ; Tp53flx/flx and _Ctnnb1-_mutant ; Tp53+/flx mice. ***=Log Rank P<0.0001. Immunoflourescence of (b) Zic1 and (c) Olig3 and Ctnnb1 expression in a _Ctnnb1-_mutant ; Tp53flx/flx medulloblastoma. (d) Hematoxylin and eosin stained low (i, v; scale=800 μm) and high (ii, vi; scale=25 μm) power views of mouse medulloblastomas and tumor-brainstem interface (iii, vii; scale bar=50μm). Ctnnb1 immunostaining (iv, viii; scale=10 μm, arrows indicate nuclear immunoreactivity). Boxes indicate location of high power views. (e) Frequency and anatomical site of mouse medulloblastomas.
Figure 4. Mutant-Ctnnb1 mouse medulloblastomas recapitulate the molecular characteristics of human WNT-subtype disease
(a) AGDEX comparison of _Ctnnb1-_mutant ; Tp53flx/flx mouse medulloblastoma, and mouse EGL, E16.5 dorsal brainstem (DBS) and human medulloblastoma subgroups. (b) Unsupervised clustering of human WNT and SHH-subtype medulloblastoma signature ortholog expression in E16.5 DBS, _Ctnnb1-_mutant ; Tp53flx/flx mouse medulloblastoma (Ctnnb1 MB), P7 GNPCs and Ptch1+/−; Tp53_−/_− medulloblastoma (Ptch1 MB). (c) Top-bottom: Nine SNP-inferred homozygous deletions in three human WNT-subtype medulloblastomas. Real-Time PCR validation of deletions in the human tumors (SD below the mean diploid copy-number). Mouse chromosomal regions syntenic for human chromosome 6. ArrayCGH-inferred copy number in _Ctnnb1-_mutant ; Tp53flx/flx mouse medulloblastomas identifies common syntenic deletion of TULP4.
Comment in
- Medulloblastoma: origins.
McCarthy N. McCarthy N. Nat Rev Cancer. 2011 Feb;11(2):79. Nat Rev Cancer. 2011. PMID: 21322837 - Medulloblastoma: origins.
McCarthy N. McCarthy N. Nat Rev Cancer. 2011 Jan 20;11(2):80. doi: 10.1038/nrc3008. Nat Rev Cancer. 2011. PMID: 21436788 No abstract available.
Similar articles
- Sonic hedgehog-associated medulloblastoma arising from the cochlear nuclei of the brainstem.
Grammel D, Warmuth-Metz M, von Bueren AO, Kool M, Pietsch T, Kretzschmar HA, Rowitch DH, Rutkowski S, Pfister SM, Schüller U. Grammel D, et al. Acta Neuropathol. 2012 Apr;123(4):601-14. doi: 10.1007/s00401-012-0961-0. Epub 2012 Feb 21. Acta Neuropathol. 2012. PMID: 22349907 - Primary Sonic Hedgehog-activated dorsal brainstem medulloblastoma and ipsilateral cerebellar atrophy in an adult.
Demir MK, Yapıcıer Ö, Mert B, Alshareefi W, Bozbuğa M. Demir MK, et al. Neuroradiol J. 2020 Feb;33(1):75-79. doi: 10.1177/1971400919892824. Epub 2019 Nov 27. Neuroradiol J. 2020. PMID: 31771412 Free PMC article. - Medulloblastoma, WNT-activated/SHH-activated: clinical impact of molecular analysis and histogenetic evaluation.
Cambruzzi E. Cambruzzi E. Childs Nerv Syst. 2018 May;34(5):809-815. doi: 10.1007/s00381-018-3765-2. Epub 2018 Mar 26. Childs Nerv Syst. 2018. PMID: 29582169 Review. - WNT/β-catenin pathway activation in Myc immortalised cerebellar progenitor cells inhibits neuronal differentiation and generates tumours resembling medulloblastoma.
Rogers HA, Sousa S, Salto C, Arenas E, Coyle B, Grundy RG. Rogers HA, et al. Br J Cancer. 2012 Sep 25;107(7):1144-52. doi: 10.1038/bjc.2012.377. Epub 2012 Aug 28. Br J Cancer. 2012. PMID: 22929883 Free PMC article. - Development and cancer of the cerebellum.
Hatten ME, Roussel MF. Hatten ME, et al. Trends Neurosci. 2011 Mar;34(3):134-42. doi: 10.1016/j.tins.2011.01.002. Trends Neurosci. 2011. PMID: 21315459 Free PMC article. Review.
Cited by
- Functional MRI Assessment of Brain Activity Patterns Associated with Reading in Medulloblastoma Survivors.
Dalboni da Rocha JL, Zou Stinnett P, Scoggins MA, McAfee SS, Conklin HM, Gajjar A, Sitaram R. Dalboni da Rocha JL, et al. Brain Sci. 2024 Sep 6;14(9):904. doi: 10.3390/brainsci14090904. Brain Sci. 2024. PMID: 39335401 Free PMC article. - PRDM6 promotes medulloblastoma by repressing chromatin accessibility and altering gene expression.
Schmidt C, Cohen S, Gudenas BL, Husain S, Carlson A, Westelman S, Wang L, Phillips JJ, Northcott PA, Weiss WA, Schwer B. Schmidt C, et al. Sci Rep. 2024 Jul 12;14(1):16074. doi: 10.1038/s41598-024-66811-6. Sci Rep. 2024. PMID: 38992221 Free PMC article. - Approaches to supratentorial brain tumours in children.
Sepulveda F, Scotto Opipari R, Coppola F, Ramaglia A, Mankad K, Alves CAP, Bison B, Löbel U. Sepulveda F, et al. Neuroradiology. 2024 Sep;66(9):1495-1512. doi: 10.1007/s00234-024-03398-9. Epub 2024 Jul 2. Neuroradiology. 2024. PMID: 38953989 Review. - Radiotherapy for Recurrent Medulloblastoma in Children and Adolescents: Survival after Re-Irradiation and First-Time Irradiation.
Adolph JE, Fleischhack G, Tschirner S, Rink L, Dittes C, Mikasch R, Dammann P, Mynarek M, Obrecht-Sturm D, Rutkowski S, Bison B, Warmuth-Metz M, Pietsch T, Pfister SM, Pajtler KW, Milde T, Kortmann RD, Dietzsch S, Timmermann B, Tippelt S; German GPOH HIT-Network. Adolph JE, et al. Cancers (Basel). 2024 May 22;16(11):1955. doi: 10.3390/cancers16111955. Cancers (Basel). 2024. PMID: 38893076 Free PMC article. - Single-Cell Chromatin Accessibility Analysis Reveals Subgroup-Specific TF-NTR Regulatory Circuits in Medulloblastoma.
Gao X, Zhuang Q, Li Y, Li G, Huang Z, Chen S, Sun S, Yang H, Jiang L, Mao Y. Gao X, et al. Adv Sci (Weinh). 2024 Aug;11(30):e2309554. doi: 10.1002/advs.202309554. Epub 2024 Jun 17. Adv Sci (Weinh). 2024. PMID: 38884167 Free PMC article.
References
- Ellison DW, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. - PubMed
- Gajjar A, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. - PubMed
- Thompson MC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. Epub 2006 Mar 1927. - PubMed
- Gilbertson RJ, Ellison DW. The Origins of Medulloblastoma Subtypes. Annu Rev Pathol. 2008;3:341–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA129541-04/CA/NCI NIH HHS/United States
- P01 CA096832-06A18120/CA/NCI NIH HHS/United States
- R01 CA129541/CA/NCI NIH HHS/United States
- R01 NS037956/NS/NINDS NIH HHS/United States
- R01 CA129541-02/CA/NCI NIH HHS/United States
- R01 CA129541-05/CA/NCI NIH HHS/United States
- R01 CA129541-03/CA/NCI NIH HHS/United States
- P30CA021765/CA/NCI NIH HHS/United States
- 01CA96832/CA/NCI NIH HHS/United States
- R01 CA129541-01/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- P01 CA096832/CA/NCI NIH HHS/United States
- R01 NS037956-13/NS/NINDS NIH HHS/United States
- P01 CA096832-078120/CA/NCI NIH HHS/United States
- R01CA129541/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous