Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro - PubMed (original) (raw)
. 2011 Feb 3;470(7332):105-9.
doi: 10.1038/nature09691. Epub 2010 Dec 12.
Christopher N Mayhew, Scott A Rankin, Matthew F Kuhar, Jefferson E Vallance, Kathryn Tolle, Elizabeth E Hoskins, Vladimir V Kalinichenko, Susanne I Wells, Aaron M Zorn, Noah F Shroyer, James M Wells
Affiliations
- PMID: 21151107
- PMCID: PMC3033971
- DOI: 10.1038/nature09691
Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro
Jason R Spence et al. Nature. 2011.
Abstract
Studies in embryonic development have guided successful efforts to direct the differentiation of human embryonic and induced pluripotent stem cells (PSCs) into specific organ cell types in vitro. For example, human PSCs have been differentiated into monolayer cultures of liver hepatocytes and pancreatic endocrine cells that have therapeutic efficacy in animal models of liver disease and diabetes, respectively. However, the generation of complex three-dimensional organ tissues in vitro remains a major challenge for translational studies. Here we establish a robust and efficient process to direct the differentiation of human PSCs into intestinal tissue in vitro using a temporal series of growth factor manipulations to mimic embryonic intestinal development. This involved activin-induced definitive endoderm formation, FGF/Wnt-induced posterior endoderm pattering, hindgut specification and morphogenesis, and a pro-intestinal culture system to promote intestinal growth, morphogenesis and cytodifferentiation. The resulting three-dimensional intestinal 'organoids' consisted of a polarized, columnar epithelium that was patterned into villus-like structures and crypt-like proliferative zones that expressed intestinal stem cell markers. The epithelium contained functional enterocytes, as well as goblet, Paneth and enteroendocrine cells. Using this culture system as a model to study human intestinal development, we identified that the combined activity of WNT3A and FGF4 is required for hindgut specification whereas FGF4 alone is sufficient to promote hindgut morphogenesis. Our data indicate that human intestinal stem cells form de novo during development. We also determined that NEUROG3, a pro-endocrine transcription factor that is mutated in enteric anendocrinosis, is both necessary and sufficient for human enteroendocrine cell development in vitro. PSC-derived human intestinal tissue should allow for unprecedented studies of human intestinal development and disease.
Conflict of interest statement
Competing interests
The authors declare no competing financial interest.
Figures
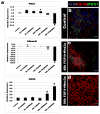
Figure 1. FGF4 and Wnt3a act synergistically in a temporal and dose-dependent manner to specify stable posterior endoderm fate
ActivinA (100ng/ml) was used to differentiate H9-HES cells into definitive endoderm (DE). DE was treated with the posteriorizing factors FGF4 (50, 500ng), Wnt3a (50, 500ng) or both for 6, 48 or 96 hours. Cells were placed in permissive media for 7 days and expression of foregut markers (ALB, PDX1) and the hindgut marker (CDX2) were analyzed by RT-qPCR (a) and immunofluorescence (b-d). Controls DE was grown for identical lengths of time in the absence of FGF4 or Wnt3a. High levels of FGF4+Wnt3a for 96 hours gave resulted in stable CDX2 expression and lack of foregut marker expression. Error bars are S.E.M (n=3). Significance is shown by; * (p<0.05), ^ (p<0.001), # (p<0.0001).
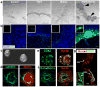
Figure 2. Morphogenesis of posterior endoderm into three-dimensional, hindgut-like spheroids
a, Bright field images of DE cultured for 96 hours in media, FGF4, Wnt3a or FGF4+Wnt3a. FGF4+Wnt3a cultures contained 3D epithelial tubes and free-floating spheres (black arrows) b, CDX2 immunostaining (Green) and nuclear stain (Draq5 - blue) on cultures shown in a. c, Bright field image of hindgut-like spheroids. d-f, Analysis of CDX2, basal-lateral laminin and E-Cadherin expression demonstrate an inner layer of polarized, cuboidal, CDX2+ epithelium surrounded by non-polarized mesenchymal CDX2+ cells. g, CDX2 expression in an e8.5 mouse embryo (sagittal section). Inset is a magnified view showing that both hindgut endoderm (E) and adjacent mesenchyme (M) are CDX2 positive (green). (FG – foregut, HG – hindgut).
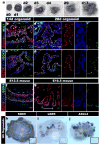
Figure 3. hESCs and hiPSCs form 3-dimensional intestine-like organoids
a, A time course shows that intestinal organoids formed highly convoluted epithelial structures surrounded by mesenchyme after 13 days. b-e, Intestinal transcription factor expression (KLF5, CDX2, SOX9) and cell proliferation on serial sections of organoids after 14 and 28 days (serial sections are b and c, d and e). f and g, Expression of KLF5, CDX2, and SOX9 in mouse fetal intestine at e14.5 (f) and e16.5 (g) is similar to developing intestinal organoids. The right panels show separate color channels for d, e and g. h, i and j, whole mount in situ hybridization of 56 day old organoids showing epithelial expression of Sox9 (h) and restricted “crypt-like” expression of the stem cell markers Lgr5 (i) and Ascl2 (j). Insets show sense controls for each probe.
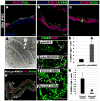
Figure 4. Formation and function of intestinal cell types and regulation of enteroendocrine differentiation by NEUROG3
28 day iPSC-derived organoids were analyzed for a, villin (VIL) and the goblet cell marker mucin (MUC2), b, the paneth cell marker lysozyme (LYSO) or c, the endocrine cell marker chromogranin A (CGA). d, Electron micrograph showing an enterocyte cell with a characteristic brush border with microvilli (inset). e, Epithelial uptake of the fluorescently labeled dipeptide d-Ala-Lys-AMCA (arrowheads) indicating a functional peptide transport system. f-h, Adenoviral expression of Neurog3 (pAd-NEUROG3) causes a 5-fold increase in CGA+ cells compared to a GFP control (pAd-GFP); (n=4 biological samples);*(p=0.005). i-k, Organoids were generated from hESCs that were stably transduced with shRNA-expressing lentiviral vectors. Compared to control shRNA organoids, NEUROG3 shRNA organoids had a 95% reduction in the number of CgA+ cells; (n=3 for shRNA controls and n=5 for Neurog3-shRNA); *(p=0.018). Error bars for h,k are S.E.M.
Comment in
- Differentiation in three dimensions.
Baker M. Baker M. Nat Methods. 2011 Feb;8(2):111. doi: 10.1038/nmeth0211-111. Nat Methods. 2011. PMID: 21355124 No abstract available. - Stem cells: Intestinal tissue generated in vitro.
Barranco C. Barranco C. Nat Rev Gastroenterol Hepatol. 2011 Feb;8(2):63. doi: 10.1038/nrgastro.2010.226. Nat Rev Gastroenterol Hepatol. 2011. PMID: 21381243 No abstract available.
References
- Spence JR, Wells JM. Translational embryology: Using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Developmental Dynamics. 2007;236:3218–3227. - PubMed
- Cai J, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–1239. - PubMed
- D’Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. - PubMed
- Song Z, et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell research. 2009;19:1233–1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 DK83202-01/DK/NIDDK NIH HHS/United States
- T32 HD007463/HD/NICHD NIH HHS/United States
- R01 CA142826/CA/NCI NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- T32 HD07463/HD/NICHD NIH HHS/United States
- R01 DK080823/DK/NIDDK NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- U54 RR025216/RR/NCRR NIH HHS/United States
- R03 DK084167/DK/NIDDK NIH HHS/United States
- R01GM072915/GM/NIGMS NIH HHS/United States
- R01DK080823A1/DK/NIDDK NIH HHS/United States
- R01 GM072915/GM/NIGMS NIH HHS/United States
- R01 DK092456/DK/NIDDK NIH HHS/United States
- F32 DK083202/DK/NIDDK NIH HHS/United States
- K01 DK091415/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials