Identification of lysine succinylation as a new post-translational modification - PubMed (original) (raw)
Identification of lysine succinylation as a new post-translational modification
Zhihong Zhang et al. Nat Chem Biol. 2011 Jan.
Abstract
Of the 20 ribosomally coded amino acid residues, lysine is the most frequently post-translationally modified, which has important functional and regulatory consequences. Here we report the identification and verification of a previously unreported form of protein post-translational modification (PTM): lysine succinylation. The succinyllysine residue was initially identified by mass spectrometry and protein sequence alignment. The identified succinyllysine peptides derived from in vivo proteins were verified by western blot analysis, in vivo labeling with isotopic succinate, MS/MS and HPLC coelution of their synthetic counterparts. We further show that lysine succinylation is evolutionarily conserved and that this PTM responds to different physiological conditions. Our study also implies that succinyl-CoA might be a cofactor for lysine succinylation. Given the apparent high abundance of lysine succinylation and the significant structural changes induced by this PTM, it is expected that lysine succinylation has important cellular functions.
Figures
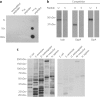
Figure 1. Illustration of chemical structures of lysine, acetyllysine, succinyllysine and methylmalonyllysine residues
The enzymatic reaction for lysine acetylation and the hypothesized mechanism for lysine succinylation are indicated.
Figure 2. Mass spectrometric identification and verification of a lysine-succinylated peptide from isocitrate dehydrogenase
(a) MS/MS spectra of a doubly charged tryptic peptide (FTEGAFKDWGYQlAR) from isocitrate dehydrogenase (top), the synthetic succinyllysine peptide corresponding to the in vivo peptide sequence (middle) and the synthetic methylmalonyllysine peptide bearing the same peptide sequence (bottom). Insets show the precursor ion masses. The neutral loss of the Co2 group is shown in red. (b) Extracted ion chromatograms (XICs) of the _in vivo_-derived isocitrate dehydrogenase peptide (top), the synthetic succinyllysine peptide bearing the same peptide sequence (middle) and a mixture of the peptide from _in vivo_-derived isocitrate dehydrogenase tryptic peptide and its synthetic counterpart (bottom). (c) The HPlC coelution profile of FTEGAFSuccKDWGYQlAR (◆) and FTEGAFMeMalKDWGYQlAR (▼), showing the different retention times of these two peptides.
Figure 3. Verification of lysine succinylation by western blot analysis
(a) Specificity of anti-SuccK antibody using dot-spot assays. Peptide libraries bearing a fixed unmodified lysine, acetyllysine or succinyllysine were spotted on nitrocellulose membrane with 10-fold dilutions. The 13-residue randomized peptide libraries have a fixed lysine residue at the seventh position: lane 1, unmodified lysine; lane 2, AcK; lane 3, SuccK. (b) Western blot analysis of recombinant E. coli isocitrate dehydrogenase (Icda), GADPH (GapA) and serine hydroxymethyltransferase (GlyA) competed with a lysine-succinylated (S) or an unmodified (U) peptide library. The same amounts of sample were loaded in both lanes for each protein. (c) Western blot analysis of lysine succinylation competed with a lysine succinylated (right) or an unmodified (left) peptide library in protein whole-cell lysates of E. coli (K-12 MG1655), S. cerevisiae (strain BY4741), D. melanogaster (S2 cells), M. musculus (C3H10 (T1/2) cells) and H. sapiens (Hela cells).
Figure 4. Mass spectrometric identification and verification of a lysine-succinylated peptide from serine hydroxymethyltransferase
(a) MS/MS spectra of a doubly charged tryptic peptide (GGSEElYKK) from serine hydromethyltransferase (top), the synthetic succinyllysine peptide corresponding to the in vivo peptide sequence (middle) and the synthetic methylmalonyllysine peptide bearing the same peptide sequence (bottom). Insets show the precursor ion masses. The neutral loss of Co2 group was shown in red. (b) XICs of the _in vivo_-derived serine hydroxymethyltransferase peptide (top), the synthetic succinyllysine peptide bearing the same peptide sequence (middle) and a mixture of the peptide from _in vivo_-derived serine hydroxymethyltransferase tryptic peptide and its synthetic counterpart (bottom). (c) The HPlC coelution profile of GGSEElYSuccKK (◆) and GGSEElYMeMalKK (▼), showing the different retention times of these two peptides.
Figure 5. Stimulation of lysine succinylation by sodium succinate and in vivo isotopic succinate labeling
(a) Stimulation of lysine succinylation in response to succinate. Western blot analysis (left) of whole-cell lysates from untreated (control), 80 mM and 160 mM sodium succinate treated (for 4 h) E. coli cells. Coomassie blue gel shows equal loading amounts (right). (b) MS/MS spectral numbers of H4- and D4-labeled succinyllysine peptides identified from E. coli that were treated with 160 mM 2,2,3,3-D4-succinate. Results were based on Mascot sequence alignment using ion score cutoff 30 and manually verified.
Figure 6. Sequence alignment and mutagenesis analysis of isocitrate dehydrogenase
(a) ClustalW (2.0.12) alignment of isocitrate dehydrogenase homologs from H. sapiens (GenInfo Identifier (GI): 5031777), M. musculus (GI: 148693874), D. melanogaster (GI: 24643268), C. elegans (GI: 71986051), S. cerevisiae (GI: 6324709) and E. coli (GI: 170080787). Conserved sites are shaded with gray and black. Conserved and nonconserved succinyllysine residues are indicated by red and blue triangles, respectively. The positions are labeled corresponding to the E. coli sequence. (b) localization of the succinyllysines and functional sites in isocitrate dehydrogenase. The three-dimensional structure was obtained from the Molecular Modeling Database (MMDB) (MMDB ID 49631) and viewed by Cn3D (v4.1). Succinylated and known functionally important sites (Uniprot ID P08200) are indicated by red and yellow arrows, respectively. The superscripts SB, NB, MB, CAT and SUCC on the labeled residues refer to: substrate binding, NADP binding, metal binding, catalytic sites and succinylation, respectively. (c) Purity of the wild-type and mutated proteins shown by SDS-PAGE gel. (d) Enzymatic activities of wild-type (control), K242R, K242E (top), K100E and K100R (bottom) isocitrate dehydrogenase mutants.
Similar articles
- Identification of lysine succinylation substrates and the succinylation regulatory enzyme CobB in Escherichia coli.
Colak G, Xie Z, Zhu AY, Dai L, Lu Z, Zhang Y, Wan X, Chen Y, Cha YH, Lin H, Zhao Y, Tan M. Colak G, et al. Mol Cell Proteomics. 2013 Dec;12(12):3509-20. doi: 10.1074/mcp.M113.031567. Epub 2013 Oct 31. Mol Cell Proteomics. 2013. PMID: 24176774 Free PMC article. - Global Analysis of Protein Lysine Succinylation Profiles and Their Overlap with Lysine Acetylation in the Marine Bacterium Vibrio parahemolyticus.
Pan J, Chen R, Li C, Li W, Ye Z. Pan J, et al. J Proteome Res. 2015 Oct 2;14(10):4309-18. doi: 10.1021/acs.jproteome.5b00485. Epub 2015 Sep 21. J Proteome Res. 2015. PMID: 26369940 - The first identification of lysine malonylation substrates and its regulatory enzyme.
Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y. Peng C, et al. Mol Cell Proteomics. 2011 Dec;10(12):M111.012658. doi: 10.1074/mcp.M111.012658. Epub 2011 Sep 9. Mol Cell Proteomics. 2011. PMID: 21908771 Free PMC article. - The growing landscape of succinylation links metabolism and heart disease.
Yang L, Miao S, Zhang J, Wang P, Liu G, Wang J. Yang L, et al. Epigenomics. 2021 Feb;13(4):319-333. doi: 10.2217/epi-2020-0273. Epub 2021 Feb 19. Epigenomics. 2021. PMID: 33605156 Review. - A review of the mechanism of succinylation in cancer.
Lu K, Han D. Lu K, et al. Medicine (Baltimore). 2022 Nov 11;101(45):e31493. doi: 10.1097/MD.0000000000031493. Medicine (Baltimore). 2022. PMID: 36397343 Free PMC article. Review.
Cited by
- Adaptative biochemical pathways and regulatory networks in Klebsiella oxytoca BAS-10 producing a biotechnologically relevant exopolysaccharide during Fe(III)-citrate fermentation.
Gallo G, Baldi F, Renzone G, Gallo M, Cordaro A, Scaloni A, Puglia AM. Gallo G, et al. Microb Cell Fact. 2012 Nov 23;11:152. doi: 10.1186/1475-2859-11-152. Microb Cell Fact. 2012. PMID: 23176641 Free PMC article. - Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines.
Gibson GE, Xu H, Chen HL, Chen W, Denton TT, Zhang S. Gibson GE, et al. J Neurochem. 2015 Jul;134(1):86-96. doi: 10.1111/jnc.13096. Epub 2015 Apr 8. J Neurochem. 2015. PMID: 25772995 Free PMC article. - Quantitative proteomic analysis of histone modifications.
Huang H, Lin S, Garcia BA, Zhao Y. Huang H, et al. Chem Rev. 2015 Mar 25;115(6):2376-418. doi: 10.1021/cr500491u. Epub 2015 Feb 17. Chem Rev. 2015. PMID: 25688442 Free PMC article. Review. No abstract available. - Mitochondrial sirtuins in the heart.
Bugger H, Witt CN, Bode C. Bugger H, et al. Heart Fail Rev. 2016 Sep;21(5):519-28. doi: 10.1007/s10741-016-9570-7. Heart Fail Rev. 2016. PMID: 27295248 Review. - Characterization and Identification of Lysine Succinylation Sites based on Deep Learning Method.
Huang KY, Hsu JB, Lee TY. Huang KY, et al. Sci Rep. 2019 Nov 7;9(1):16175. doi: 10.1038/s41598-019-52552-4. Sci Rep. 2019. PMID: 31700141 Free PMC article.
References
- Walsh CT, Garneau-Tsodikova S, Gatto GJ., Jr. Protein post-translational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Edn. Engl. 2005;44:7342–7372. - PubMed
- Witze ES, Old WM, Resing KA, Ahn NG. Mapping protein post-translational modifications with mass spectrometry. Nat. Methods. 2007;4:798–806. - PubMed
- Hake SB, Xiao A, Allis CD. Linking the epigenetic `language' of covalent histone modifications to cancer. Br. J. Cancer. 2007;96(Suppl):R31–R39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials