Development and function of CD94-deficient natural killer cells - PubMed (original) (raw)
Development and function of CD94-deficient natural killer cells
Mark T Orr et al. PLoS One. 2010.
Abstract
The CD94 transmembrane-anchored glycoprotein forms disulfide-bonded heterodimers with the NKG2A subunit to form an inhibitory receptor or with the NKG2C or NKG2E subunits to assemble a receptor complex with activating DAP12 signaling proteins. CD94 receptors expressed on human and mouse NK cells and T cells have been proposed to be important in NK cell tolerance to self, play an important role in NK cell development, and contribute to NK cell-mediated immunity to certain infections including human cytomegalovirus. We generated a gene-targeted CD94-deficient mouse to understand the role of CD94 receptors in NK cell biology. CD94-deficient NK cells develop normally and efficiently kill NK cell-susceptible targets. Lack of these CD94 receptors does not alter control of mouse cytomegalovirus, lymphocytic choriomeningitis virus, vaccinia virus, or Listeria monocytogenes. Thus, the expression of CD94 and its associated NKG2A, NKG2C, and NKG2E subunits is dispensable for NK cell development, education, and many NK cell functions.
Conflict of interest statement
Competing Interests: The authors have read the journal's policy and have the following conflicts: J.H.P. is an employee of Merck; P.S. and T.E. are employees of Novo Nordisk. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials. The other authors have no financial conflicts of interest.
Figures
Figure 1. Splenic CD94-deficient and CD94Tg/– NK cells are phenotypically normal.
(A) Splenocytes from B6, CD94-deficient, CD94Tg/–, and 129/SvJ mice were analyzed for CD94-NKG2 expression. CD94-NKG2+ cells were analyzed for NKp46 and TCRβ expression as shown in the second column. (B) NK cells (NKp46+ TCRβ–) were analyzed for expression of CD94, NKG2A/C/E, NK1.1, Ly49C/I, and Ly49H. (C) CD19– bone marrow cells were analyzed for NK cell precursors (NKG2D+ CD122+) and the developmental markers DX5, αV, CD27, and CD11b. Data are representative of three to five experiments each.
Figure 2. CD94-deficient NK cells are educated and efficiently kill YAC-1 targets and MHC I-deficient splenocytes.
(A) Splenocytes from B6, CD94-deficient, CD94Tg/–, and 129/SvJ mice were assayed for degranulation as measured by surface staining for CD107a and intracellular IFN-γ production upon stimulated with plate-bound anti-NKp46 mAb. (B) Splenocytes from CD94-deficient mice and wildtype 129/SvJ mice primed with poly I:C in vivo were assayed for NK cell-mediated cytotoxicity against the YAC-1 target cell. (C) CD94-deficient mice and B6 mice either depleted of NK cells with anti-NK1.1 mAb or treated with PBS received a mixture of CFSE-labeled wildtype and B2m−/− B6 splenocytes. Twenty-four hours later the frequency or CFSE-labeled donor cells in the spleen that were _B2m−/−_was determined by expression of H-2Kb. Data are representative of three to five experiments each.
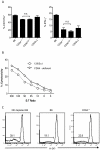
Figure 3. CD94 is not necessary for control of MCMV.
(A) Two days after infection with MCMV splenic NK cells from B6 and CD94-deficient mice were analyzed for expression of CD69 and intracellular IFN-γ and granzyme B. (B) CD94-deficient and CD94Tg/– mice, (C) B6 mice treated with the non-depleting, blocking anti-NKG2A/C/E chimeric rat-mouse monoclonal antibody 20D5HCmIgG1-Q, anti-NK1.1 depleting antibody or PBS (D), or BALB/c mice receiving the 20D5HCmIgG1-Q antibody or PBS were infected with 5×104 pfu MCMV and then analyzed for viral titers three days later. Graphs represent the average ± s.e.m. of four or five animals per group. Not statistically significant (n.s.).
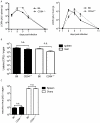
Figure 4. CD94 is not necessary for clearance of LCMV, vaccinia virus or Listeria infection.
(A) B6 and CD94-deficient mice were infected with 2×105 pfu of LCMV (Armstrong strain). Spleens and livers were analyzed for LCMV titers on the indicated days. (B) B6 and CD94-deficient mice were challenged with 1×105 cfu Listeria monocytogenes. Spleens and livers were analyzed for bacterial burdens three days later. (C) B6 and CD94-deficient mice were challenged with 5×106 pfu vaccinia virus. Spleens and ovaries were analyzed for viral titers seven days later. Graphs represent the average ± s.e.m. of three to five animals per group per time point.
Similar articles
- Rat and mouse CD94 associate directly with the activating transmembrane adaptor proteins DAP12 and DAP10 and activate NK cell cytotoxicity.
Saether PC, Hoelsbrekken SE, Fossum S, Dissen E. Saether PC, et al. J Immunol. 2011 Dec 15;187(12):6365-73. doi: 10.4049/jimmunol.1102345. Epub 2011 Nov 14. J Immunol. 2011. PMID: 22084441 - Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts.
Gumá M, Budt M, Sáez A, Brckalo T, Hengel H, Angulo A, López-Botet M. Gumá M, et al. Blood. 2006 May 1;107(9):3624-31. doi: 10.1182/blood-2005-09-3682. Epub 2005 Dec 29. Blood. 2006. PMID: 16384928 - IL-12-dependent inducible expression of the CD94/NKG2A inhibitory receptor regulates CD94/NKG2C+ NK cell function.
Sáez-Borderías A, Romo N, Magri G, Gumá M, Angulo A, López-Botet M. Sáez-Borderías A, et al. J Immunol. 2009 Jan 15;182(2):829-36. doi: 10.4049/jimmunol.182.2.829. J Immunol. 2009. PMID: 19124726 - The CD94/NKG2C+ NK-cell subset on the edge of innate and adaptive immunity to human cytomegalovirus infection.
López-Botet M, Muntasell A, Vilches C. López-Botet M, et al. Semin Immunol. 2014 Apr;26(2):145-51. doi: 10.1016/j.smim.2014.03.002. Epub 2014 Mar 22. Semin Immunol. 2014. PMID: 24666761 Review. - Endocytosis as a mechanism of regulating natural killer cell function: unique endocytic and trafficking pathway for CD94/NKG2A.
Peruzzi G, Masilamani M, Borrego F, Coligan JE. Peruzzi G, et al. Immunol Res. 2009;43(1-3):210-22. doi: 10.1007/s12026-008-8072-7. Immunol Res. 2009. PMID: 18979076 Free PMC article. Review.
Cited by
- Feeder-free differentiation of human iPSCs into natural killer cells with cytotoxic potential against malignant brain rhabdoid tumor cells.
Kiran S, Xue Y, Sarker DB, Li Y, Sang QA. Kiran S, et al. Bioact Mater. 2024 Mar 8;36:301-316. doi: 10.1016/j.bioactmat.2024.02.031. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38496035 Free PMC article. - Dimerization of Transmembrane Proteins in Cancer Immunotherapy.
Li L, Li J. Li L, et al. Membranes (Basel). 2023 Mar 30;13(4):393. doi: 10.3390/membranes13040393. Membranes (Basel). 2023. PMID: 37103820 Free PMC article. Review. - Epigenetic control of CD1D expression as a mechanism of resistance to immune checkpoint therapy in poorly immunogenic melanomas.
Wang MM, Koskela SA, Mehmood A, Langguth M, Maranou E, Figueiredo CR. Wang MM, et al. Front Immunol. 2023 Apr 3;14:1152228. doi: 10.3389/fimmu.2023.1152228. eCollection 2023. Front Immunol. 2023. PMID: 37077920 Free PMC article. - Disruption of the NKG2A:HLA-E Immune Checkpoint Axis to Enhance NK Cell Activation against Cancer.
Fisher JG, Doyle ADP, Graham LV, Khakoo SI, Blunt MD. Fisher JG, et al. Vaccines (Basel). 2022 Nov 23;10(12):1993. doi: 10.3390/vaccines10121993. Vaccines (Basel). 2022. PMID: 36560403 Free PMC article. Review. - The transcription factor Bach2 negatively regulates murine natural killer cell maturation and function.
Li S, Bern MD, Miao B, Fan C, Xing X, Inoue T, Piersma SJ, Wang T, Colonna M, Kurosaki T, Yokoyama WM. Li S, et al. Elife. 2022 Oct 3;11:e77294. doi: 10.7554/eLife.77294. Elife. 2022. PMID: 36190189 Free PMC article.
References
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. - PubMed
- Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. - PubMed
- Gazit R, Gruda R, Elboim M, Arnon TI, Katz G, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7:517–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21AI077021/AI/NIAID NIH HHS/United States
- R21 AI077021/AI/NIAID NIH HHS/United States
- AI066897/AI/NIAID NIH HHS/United States
- CA006927/CA/NCI NIH HHS/United States
- R01 AI065544/AI/NIAID NIH HHS/United States
- R37 AI066897/AI/NIAID NIH HHS/United States
- R01AI065544/AI/NIAID NIH HHS/United States
- P30 CA006927/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous