Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity - PubMed (original) (raw)
doi: 10.1172/JCI44845. Epub 2010 Dec 13.
Hye Young Kim, Lee A Albacker, Hyun Hee Lee, Nicole Baumgarth, Shizuo Akira, Paul B Savage, Shin Endo, Takashi Yamamura, Janneke Maaskant, Naoki Kitano, Abel Singh, Apoorva Bhatt, Gurdyal S Besra, Peter van den Elzen, Ben Appelmelk, Richard W Franck, Guangwu Chen, Rosemarie H DeKruyff, Michio Shimamura, Petr Illarionov, Dale T Umetsu
Affiliations
- PMID: 21157038
- PMCID: PMC3007162
- DOI: 10.1172/JCI44845
Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity
Ya-Jen Chang et al. J Clin Invest. 2011 Jan.
Abstract
Infection with influenza A virus represents a major public health threat worldwide, particularly in patients with asthma. However, immunity induced by influenza A virus may have beneficial effects, particularly in young children, that might protect against the later development of asthma, as suggested by the hygiene hypothesis. Herein, we show that infection of suckling mice with influenza A virus protected the mice as adults against allergen-induced airway hyperreactivity (AHR), a cardinal feature of asthma. The protective effect was associated with the preferential expansion of CD4-CD8-, but not CD4+, NKT cells and required T-bet and TLR7. Adoptive transfer of this cell population into allergen-sensitized adult mice suppressed the development of allergen-induced AHR, an effect associated with expansion of the allergen-specific forkhead box p3+ (Foxp3+) Treg cell population. Influenza-induced protection was mimicked by treating suckling mice with a glycolipid derived from Helicobacter pylori (a bacterium associated with protection against asthma) that activated NKT cells in a CD1d-restricted fashion. These findings suggest what we believe to be a novel pathway that can regulate AHR, and a new therapeutic strategy (treatment with glycolipid activators of this NKT cell population) for asthma.
Figures
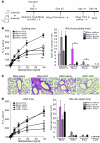
Figure 1. Infection of suckling mice with H3N1 protects the mice against AHR when adults.
(A) Schematic showing the protocol for OVA-induced AHR. Two-week-old (suckling) or 8 week-old (adult) mice were treated with influenza A virus (H3N1) or control AF (mock infection) and assessed 6 weeks later as adults for AHR. (B) BALB/c mice (n = 8 per group) treated with H3N1 or AF at 2 weeks of age were assessed 42 days after infection for OVA-induced AHR. Changes in lung resistance (RL) were measured in anesthetized, tracheotomized, intubated, and mechanically ventilated mice (left panel). ***P < 0.001 compared with mock-infected group. Cells in BAL were collected and analyzed 24 hours after the final OVA challenge (right panel). *P < 0.05 compared with mock-infected group. (C) Representative lung sections stained with H&E (original magnification, ×10) from mock- or H3N1-infected mice treated with saline or challenged with OVA. (D) Eight-week-old BALB/c mice (n = 5 per group) were infected with H3N1 or AF. Six weeks after infection, the mice were assessed for OVA-induced AHR by measuring lung resistance (left panel). Cells in BAL were collected and analyzed 24 hours after the final OVA challenge (right panel). Data are representative of 3 independent experiments.
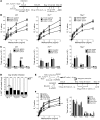
Figure 2. Adoptive transfer of H3N1-exposed NKT cells fails to reconstitute OVA-induced AHR.
(A) Schematic showing the protocol for adoptive transfer of NKT cells to OVA-immunized J_α_18–/– recipients. The donor mice were infected with H3N1 or mock infected at 2 weeks of age. Six weeks after infection, NKT cells were purified and adoptively transferred into OVA-sensitized J_α_18–/– mice, which were then challenged with OVA and assessed for AHR. (B) Adoptive transfer of H3N1-exposed NKT cells (vNKT) to J_α_18–/– mice failed to reconstitute OVA-induced AHR (measured as lung resistance in response to methacholine challenge) (left panel). Adoptive transfer of NKT cells from mock-infected mice (NKT) fully reconstituted AHR. H3N1 infection at 2 weeks of age of J_α_18–/– mice (v_J_α_18–/–_) and reconstitution at 8 weeks of age with NKT cells from mock-infected mice did not protect against AHR (n = 8–10 per group). BAL fluid was collected and analyzed (right panel). *P < 0.05 and **P < 0.01, compared with J_α_18–/– + NKT group. (C and D) Lung cells were isolated from the recipients after measurement of AHR, and the absolute numbers (C) and percentages (D) of lung CD4+ or CD4–CD8– (DN) NKT subsets were assessed by FACS. Upper panels show dot plots for NKT cells in lung leukocytes. After gating on the NKT cells, the cells were analyzed for CD4 and CD8 (lower panels). ***P < 0.001 compared with WT NKT group. Data are representative of 3 independent experiments.
Figure 3. H3N1 infection in 2-week-old mice alters the phenotype of the NKT cells.
(A) Lung cells were isolated over a 6-week period and analyzed for NKT cells. Left: Absolute numbers of lung NKT cells. Right: Percentage of NKTs (top) in lung leukocytes. NKT cells were analyzed for CD4 and CD8 (bottom). (B) Left: BALB/c mice (n = 3/group) were infected with H3N1 or AF at 2 or 8 weeks of age, and lung NKT cells were assessed over 2 weeks. Right: Percentage of NKT cells in lungs of 2-week-old and 8-week-old mice. (C) Two-week-old BALB/c mice were mock infected or infected with H3N1, and pulmonary CD4+ NKT and DN NKT cell numbers were assessed on days 1 and 14 after infection. (D and E) NKT cells from C were assessed for CD4, IFN-γ, and IL-4 expression (D) and absolute numbers quantified (E). (F) BALB/c mice (n = 4–5/group) were infected with H3N1 or mock infected at 2 or 8 weeks of age, and lung samples were taken 42 days later to assess NKT cell subsets. One of 2 independent experiments is shown. (G) Two-week-old BALB/c mice were infected with H3N1 or mock infected. After 42 days, lung cells were harvested and stimulated ex vivo with vehicle or α-GalCer for 96 hours. IFN-γ and IL-4 in supernatants from triplicate wells were determined by ELISA and the IFN-γ/IL-4 ratio calculated. *P < 0.05, ***P < 0.001 compared with mock infection.
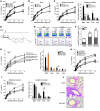
Figure 4. H3N1-exposed NKT cells suppress AHR and increase OVA-specific Tregs.
(A) Protocol for adoptive transfer of NKT cells. (B and C) Lung resistance was measured in recipient mice (B; n = 15/group) and BAL cells collected (C). (D) Relative numbers of CD4+ versus DN NKT cells in recipients’ lungs were assessed (E) H3N1-exposed CD4–CD8–NKT (vDN NKT) or CD4+NKT (vCD4 NKT) cells were purified and transferred as in A. Lung resistance was measured in recipient mice (n = 5/group). (F) Eight-week-old WT BALB/c mice received 5 × 104 DO11.10 Rag–/– T cells and were sensitized with OVA/alum. Seven days later, NKT cells from WT BALB/c, Vα14tg, or H3N1-infected mice were adoptively transferred into OVA-sensitized mice. After OVA challenge, the numbers of natural Tregs (CD4+C25+Foxp3+) and adaptive OVA antigen–specific Tregs (CD4+ CD25+ Foxp3+KJ1-26+) were determined. Absolute cell numbers were calculated (n = 5/group). (G) Eight-week-old WT BALB/c recipients were depleted of Tregs through injections of anti-CD25 mAb (clone PC61; 0.5 mg) and assessed as in A (n = 5/group). (H and I) NKT cells from WT or Vα14 Tg were transferred to OVA-sensitized BALB/c mice (n = 4–6/group), which were assessed as in A (H), and BAL cells were analyzed (I). (J) Representative lung sections from recipients described in H were H&E stained (original magnification, ×10). Data represent 2–3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus WT NKT-OVA (B–D), OVA (E), WT NKT (F, H, and I), and OVA-vNKT (G).
Figure 5. The protective effect of H3N1 infection depends on TLR7 and T-bet.
(A) Schematic showing the protocol for WT, Tlr7–/–, or Tbet–/– mice infected at 2 weeks of age with H3N1 virus or mock infected and examined for OVA-induced AHR at 8 weeks of age (n = 4–6 per group). (B) Lung resistance was measured. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the mock-OVA group. (C) BAL cells from B were collected. (D) WT, Tlr7–/–, or Tbet–/– mice were infected with H3N1 or mock at 2 weeks of age, and lung samples were taken 42 days later to asses for NKT cell subsets. ***P < 0.001 compared with the mock group. (E) Schematic showing the adoptive transfer of NKT from virus-infected WT, Tlr7–/–, or Tbet–/– mice to OVA-sensitized BALB/c recipients (n = 4–6 per group). The donor mice were infected with H3N1 or mock-infected at 2 weeks of age. NKT cells were purified from these mice 42 days after infection and transferred to OVA-sensitized BALB/c mice, which were then challenged with OVA to induce AHR. (F) Left: After OVA challenge, AHR was measured as described in D. Right: Cells in BAL were assessed. ***P < 0.001 compared with the WT-OVA group. Data are representative of 2 independent experiments.
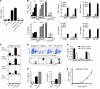
Figure 6. Induction of protection with α-C-GalCer and a glycolipid from H. pylori.
(A) Two-week-old BALB/c mice (n = 6–8/group) or (B) Tbet–/– mice (n = 4–6 per group) received 5 μg α-GalCer (cGal), 2 μg α-GalCer, or vehicle. After OVA sensitization and challenge, AHR was measured on day 44. (C) Donor mice were treated with α-C-GalCer (5 μg) or vehicle i.p. NKT cells served as donors, as in Figure 4A (n = 4 per group). Lung resistance (left) and cell counts in BAL (right) were assessed. (D) Structure of PI57. (E) Mice received PI57 (50 μg), α-GalCer (2 μg), or vehicle i.p., and lungs were examined 1 or 14 days later for CD4 and CD8 expression. (F) Absolute numbers of CD4+ NKT and DN NKT subsets from E were assessed. (G) BALB/c mice (n = 5–8/group) received PI57 or vehicle i.p. Lung resistance (left) and BAL cells (right) were assessed. (H) BALB/c mice treated with PI57 (50 μg), PBS30 (Sphingomonas glycolipid) (50 μg), or vehicle i.p. were assessed for AHR as in G. (I) Donor mice were treated with PI57 (50 μg) or vehicle i.p. NKT cells served as donors as in Figure 4A. Lung resistance (left) and BAL cells (right) were assessed (n = 4 per group). (J) Representative lung sections from I stained with H&E (original magnification, ×10). Data represent 2–3 independent experiments. *P < 0.05, #P < 0.05, ***P < 0.001 versus vehicle-OVA (C, G, and I), DN NKT saline (F), and CD4+ NKT saline (F).
Figure 7. PI57 directly activates NKT cells.
(A) NKT cell lines were cocultured with BM-derived DCs (BMDCs) and α-GalCer (100 ng/ml), PI57 (10 μg/ml), or vehicle for 48 hours, with or without pre-incubation with anti-CD1d (10 μg/ml). IFN-γ was measured by ELISA. (B) Murine NKT cell lines were cocultured as in A with BMDCs from WT, Cd1d–/–, Myd88–/–, or Trif–/– mice. Cells were treated with α-GalCer (100 ng/ml), PI57 (2.5, 5, or 10 μg/ml), PBS30 (1, 2.5, or 5 μg/ml), or vehicle for 48 hours. IFN-γ and IL-4 were measured by ELISA. (C) IL-2 production from hybridomas derived from invariant Vα14 NKT cells (RT2, RT23, and RT24) and an irrelevant Vβ8+ T cell (RT8; control) (see Supplemental Methods). (D) Mouse NKT cell lines were stained with PE-labeled CD1d tetramers of PI57 or α-GalCer at 4°C for 45 minutes or 37°C for 25 minutes, and with anti-TCRβ–APC antibody. Top: Lymphocytes were gated in the FSC/SSC window. Bottom: Percentage of CD1d tetramer+ cells. (E) IFN-γ and IL-4 production from human NKT cell lines by treatment with α-GalCer (100 ng/ml), PI57 (10 μg/ml), or vehicle for 48 hours in vitro (see Supplemental Methods). (F) IFN-γ production from CD1d-transfected NKT cell clone BM2a.3 in presence of PI57 and blocking mAb against human CD1d or CD1b (see Supplemental Methods). (G) CD1d Fc-coated Maxisorp plates were loaded with lipid and cultured with 5 × 104 NKT cells. IFN-γ was analyzed by ELISA after 24 hours. Data represent 3 or 5 independent experiments.
Similar articles
- A natural killer T-cell subset that protects against airway hyperreactivity.
Chuang YT, Leung K, Chang YJ, DeKruyff RH, Savage PB, Cruse R, Benoit C, Elewaut D, Baumgarth N, Umetsu DT. Chuang YT, et al. J Allergy Clin Immunol. 2019 Feb;143(2):565-576.e7. doi: 10.1016/j.jaci.2018.03.022. Epub 2018 May 29. J Allergy Clin Immunol. 2019. PMID: 29852257 - Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity.
Kim HY, Chang YJ, Subramanian S, Lee HH, Albacker LA, Matangkasombut P, Savage PB, McKenzie AN, Smith DE, Rottman JB, DeKruyff RH, Umetsu DT. Kim HY, et al. J Allergy Clin Immunol. 2012 Jan;129(1):216-27.e1-6. doi: 10.1016/j.jaci.2011.10.036. Epub 2011 Nov 25. J Allergy Clin Immunol. 2012. PMID: 22119406 Free PMC article. - Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells.
Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Meyer EH, et al. Proc Natl Acad Sci U S A. 2006 Feb 21;103(8):2782-7. doi: 10.1073/pnas.0510282103. Epub 2006 Feb 14. Proc Natl Acad Sci U S A. 2006. PMID: 16478801 Free PMC article. - Respiratory infection with influenza A virus interferes with the induction of tolerance to aeroallergens.
Tsitoura DC, Kim S, Dabbagh K, Berry G, Lewis DB, Umetsu DT. Tsitoura DC, et al. J Immunol. 2000 Sep 15;165(6):3484-91. doi: 10.4049/jimmunol.165.6.3484. J Immunol. 2000. PMID: 10975869 - iNKT cells in allergic disease.
Meyer EH, DeKruyff RH, Umetsu DT. Meyer EH, et al. Curr Top Microbiol Immunol. 2007;314:269-91. doi: 10.1007/978-3-540-69511-0_11. Curr Top Microbiol Immunol. 2007. PMID: 17593665 Review.
Cited by
- Activation strategies for invariant natural killer T cells.
Kohlgruber AC, Donado CA, LaMarche NM, Brenner MB, Brennan PJ. Kohlgruber AC, et al. Immunogenetics. 2016 Aug;68(8):649-63. doi: 10.1007/s00251-016-0944-8. Epub 2016 Jul 25. Immunogenetics. 2016. PMID: 27457886 Free PMC article. Review. - Control of intestinal homeostasis through crosstalk between natural killer T cells and the intestinal microbiota.
Dowds CM, Blumberg RS, Zeissig S. Dowds CM, et al. Clin Immunol. 2015 Aug;159(2):128-33. doi: 10.1016/j.clim.2015.05.008. Epub 2015 May 16. Clin Immunol. 2015. PMID: 25988859 Free PMC article. Review. - CD8+ T-cell immunity orchestrated by iNKT cells.
Qin Y, Bao X, Zheng M. Qin Y, et al. Front Immunol. 2023 Jan 18;13:1109347. doi: 10.3389/fimmu.2022.1109347. eCollection 2022. Front Immunol. 2023. PMID: 36741397 Free PMC article. Review. - Role of CD1d- and MR1-Restricted T Cells in Asthma.
Iwamura C, Nakayama T. Iwamura C, et al. Front Immunol. 2018 Aug 28;9:1942. doi: 10.3389/fimmu.2018.01942. eCollection 2018. Front Immunol. 2018. PMID: 30210497 Free PMC article. Review. - The split personality of NKT cells in malignancy, autoimmune and allergic disorders.
Subleski JJ, Jiang Q, Weiss JM, Wiltrout RH. Subleski JJ, et al. Immunotherapy. 2011 Oct;3(10):1167-84. doi: 10.2217/imt.11.117. Immunotherapy. 2011. PMID: 21995570 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL069507/HL/NHLBI NIH HHS/United States
- RC1 HL069507/HL/NHLBI NIH HHS/United States
- R01 AI026322/AI/NIAID NIH HHS/United States
- R01 HL062348/HL/NHLBI NIH HHS/United States
- R01 HL62348/HL/NHLBI NIH HHS/United States
- R01 AI68085/AI/NIAID NIH HHS/United States
- R01 AI068085/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials