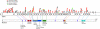Mutations in the CHD7 gene: the experience of a commercial laboratory - PubMed (original) (raw)
Mutations in the CHD7 gene: the experience of a commercial laboratory
Cynthia F Bartels et al. Genet Test Mol Biomarkers. 2010 Dec.
Abstract
CHARGE syndrome is an autosomal dominant multisystem disorder caused by mutation in the CHD7 gene, encoding chromodomain helicase DNA-binding protein 7. Molecular diagnostic testing for CHD7 mutation has been available in a clinical setting since 2005. We report here the results from the first 642 unrelated proband samples submitted for testing. Thirty-two percent (n = 203) of patient samples had a heterozygous pathogenic variant identified. The lower mutation rate than that published for well-characterized clinical samples is likely due to referral bias, as samples submitted for clinical testing may be for "rule-out" diagnoses, rather than solely to confirm clinical suspicion. We identified 159 unique pathogenic mutations, and of these, 134 mutations were each seen in a single individual and 25 mutations were found in two to five individuals (n =69). Of the 203 mutations, only 9 were missense, with 107 nonsense, 69 frameshift, and 15 splice-site mutations likely leading to haploinsufficiency at the cellular level. An additional 72 variations identified in the 642 tested samples (11%) were considered to have unknown clinical significance. Copy number changes (deletion/duplication of the entire gene or one/several exons) were found to account for a very small number of cases (n = 3). This cohort represents the largest CHARGE syndrome sample size to date and is intended to serve as a resource for clinicians, genetic counselors, researchers, and other diagnostic laboratories.
Figures
FIG. 1.
The CHD7 gene (top) and protein (bottom). Colored circles above exons 2–38 depict the location of 200 CHARGE-causing mutations. Overlapping circles indicate identical mutations. Protein domains are labeled and lines indicate where each protein domain is encoded on the gene. All DNA mutations that introduced stop codons or frameshifts were considered disease causing, as were mutations of the canonical splice donor–acceptor pair (GT-AG). Missense changes or other putative splicing changes were not considered disease causing unless the change was a de novo mutation not found in either parent or if it was reported in the literature as a de novo mutation.
Similar articles
- Mutation update on the CHD7 gene involved in CHARGE syndrome.
Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, Hofstra RM, van Ravenswaaij-Arts CM, Hoefsloot LH. Janssen N, et al. Hum Mutat. 2012 Aug;33(8):1149-60. doi: 10.1002/humu.22086. Epub 2012 Apr 16. Hum Mutat. 2012. PMID: 22461308 Review. - Complete screening of 50 patients with CHARGE syndrome for anomalies in the CHD7 gene using a denaturing high-performance liquid chromatography-based protocol: new guidelines and a proposal for routine diagnosis.
Bilan F, Legendre M, Charraud V, Manière B, Couet D, Gilbert-Dussardier B, Kitzis A. Bilan F, et al. J Mol Diagn. 2012 Jan;14(1):46-55. doi: 10.1016/j.jmoldx.2011.08.003. Epub 2011 Oct 25. J Mol Diagn. 2012. PMID: 22033296 - Identification of one novel CHD7 mutation in a patient from China with atypical CHARGE syndrome.
Cheng J, Ma D, Wu Y, Luo C, Huang C, Hu P, Zhang J, Jiang T, Xu Z. Cheng J, et al. Gene. 2015 Oct 25;571(2):298-302. doi: 10.1016/j.gene.2015.07.042. Epub 2015 Jul 15. Gene. 2015. PMID: 26187070 - Non-homologous end joining repair mechanism-mediated deletion of CHD7 gene in a patient with typical CHARGE syndrome.
Lee SJ, Chae JH, Lee JA, Cho SI, Seo SH, Park H, Seong MW, Park SS. Lee SJ, et al. Ann Lab Med. 2015 Jan;35(1):141-5. doi: 10.3343/alm.2015.35.1.141. Epub 2014 Dec 8. Ann Lab Med. 2015. PMID: 25553296 Free PMC article. - CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype.
Bergman JE, Janssen N, Hoefsloot LH, Jongmans MC, Hofstra RM, van Ravenswaaij-Arts CM. Bergman JE, et al. J Med Genet. 2011 May;48(5):334-42. doi: 10.1136/jmg.2010.087106. Epub 2011 Mar 4. J Med Genet. 2011. PMID: 21378379 Review.
Cited by
- Microglial depletion after brain injury prolongs inflammation and impairs brain repair, adult neurogenesis and pro-regenerative signaling.
Palsamy K, Chen JY, Skaggs K, Qadeer Y, Connors M, Cutler N, Richmond J, Kommidi V, Poles A, Affrunti D, Powell C, Goldman D, Parent JM. Palsamy K, et al. Glia. 2023 Nov;71(11):2642-2663. doi: 10.1002/glia.24444. Epub 2023 Jul 14. Glia. 2023. PMID: 37449457 Free PMC article. - Genetic Analysis of Patients with Congenital Hypogonadotropic Hypogonadism: A Case Series.
Cannarella R, Gusmano C, Condorelli RA, Bernini A, Kaftalli J, Maltese PE, Paolacci S, Dautaj A, Marceddu G, Bertelli M, La Vignera S, Calogero AE. Cannarella R, et al. Int J Mol Sci. 2023 Apr 18;24(8):7428. doi: 10.3390/ijms24087428. Int J Mol Sci. 2023. PMID: 37108593 Free PMC article. - Whole-Exome Sequencing Identified Rare Genetic Variants Associated with Undervirilized Genitalia in Taiwanese Pediatric Patients.
Tsai MC, Weng YH, Lin YF, Wang YC, Yu HW, Chou YY, Chen PC. Tsai MC, et al. Biomedicines. 2023 Jan 17;11(2):242. doi: 10.3390/biomedicines11020242. Biomedicines. 2023. PMID: 36830778 Free PMC article. - Novel Genomic Variants, Atypical Phenotypes and Evidence of a Digenic/Oligogenic Contribution to Disorders/Differences of Sex Development in a Large North African Cohort.
Zidoune H, Ladjouze A, Chellat-Rezgoune D, Boukri A, Dib SA, Nouri N, Tebibel M, Sifi K, Abadi N, Satta D, Benelmadani Y, Bignon-Topalovic J, El-Zaiat-Munsch M, Bashamboo A, McElreavey K. Zidoune H, et al. Front Genet. 2022 Aug 30;13:900574. doi: 10.3389/fgene.2022.900574. eCollection 2022. Front Genet. 2022. PMID: 36110220 Free PMC article. - New findings in oligogenic inheritance of congenital hypogonadotropic hypogonadism.
Gach A, Pinkier I, Wysocka U, Sałacińska K, Salachna D, Szarras-Czapnik M, Pietrzyk A, Sakowicz A, Nykel A, Rutkowska L, Rybak-Krzyszkowska M, Socha M, Jamsheer A, Jakubowski L. Gach A, et al. Arch Med Sci. 2020 Sep 18;18(2):353-364. doi: 10.5114/aoms.2020.98909. eCollection 2022. Arch Med Sci. 2020. PMID: 35316923 Free PMC article.
References
- Antonarakis SE. Krawczak M. Cooper DN. Disease-causing mutations in the human genome. Eur J Pediatr. 2000;159(Suppl 3):S173–S178. - PubMed
- Aramaki M. Kimura T. Udaka T, et al. Embryonic expression profile of chicken CHD7, the ortholog of the causative gene for CHARGE syndrome. Birth Defects Res A Clin Mol Teratol. 2007;79:50–57. - PubMed
- Aramaki M. Udaka T. Kosaki R, et al. Phenotypic spectrum of CHARGE syndrome with CHD7 mutations. J Pediatr. 2006;148:410–414. - PubMed
- Asakura Y. Toyota Y. Muroya K, et al. Endocrine and radiological studies in patients with molecularly confirmed CHARGE syndrome. J Clin Endocrinol Metab. 2008;93:920–924. - PubMed
- Bergman JE. de Wijs I. Jongmans MC, et al. Exon copy number alterations of the CHD7 gene are not a major cause of CHARGE and CHARGE-like syndrome. Eur J Med Genet. 2008;51:417–425. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases