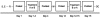Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans - PubMed (original) (raw)
Comparative Study
Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans
Shelley L Amen et al. Neuropsychopharmacology. 2011 Mar.
Abstract
Addiction is a chronic relapsing disorder hypothesized to be produced by drug-induced plasticity that renders individuals vulnerable to craving-inducing stimuli such as re-exposure to the drug of abuse. Drug-induced plasticity that may result in the addiction phenotype includes increased excitatory signaling within corticostriatal pathways that correlates with craving in humans and is necessary for reinstatement in rodents. Reduced cystine-glutamate exchange by system x(c)- appears to contribute to heightened excitatory signaling within the striatum, thereby posing this as a novel target in the treatment of addiction. In the present report, we examined the impact of repeated N-acetyl cysteine, which is commonly used to activate cystine-glutamate exchange, on reinstatement in rodents in a preclinical study and on craving in cocaine-dependent humans in a preliminary, proof-of-concept clinical experiment. Interestingly, repeated administration (7 days) of N-acetyl cysteine (60 mg/kg, IP) produced a significant reduction in cocaine (10 mg/kg, IP)-induced reinstatement, even though rats (N=10-12/group) were tested 24 h after the last administration of N-acetyl cysteine. The reduction in behavior despite the absence of the N-acetyl cysteine indicates that repeated N-acetyl cysteine may have altered drug-induced plasticity that underlies drug-seeking behavior. In parallel, our preliminary clinical data indicate that repeated administration (4 days) of N-acetyl cysteine (1200-2400 mg/day) to cocaine-dependent human subjects (N=4 per group) produced a significant reduction in craving following an experimenter-delivered IV injection of cocaine (20 mg/70 kg/60 s). Collectively, these data demonstrate that N-acetyl cysteine diminishes the motivational qualities of a cocaine challenge injection possibly by altering pathogenic drug-induced plasticity.
Figures
Figure 1
Participants underwent phone and clinical interview/laboratory screening (S) before study enrollment (E). After 1 to 8 weeks, participants underwent a run-up procedure (R) to determine the safety of delivering cocaine (20 mg per 70 kg IV over 60 s). Afterwards, participants remained inpatient for 1 to 2 separate 4-day/3-night stays, each with one blinded, oral study medication: treatment A (oral _N_-acetyl cysteine; 400 or 800 mg TID) or B (oral baclofen; 20 mg TID). During each inpatient visit, the participant underwent a 1-h test session before and after drug treatment (pretest and posttest, respectively) while receiving a total of nine doses of the test medication during the 3 days between the two tests. Participants were discharged home on day 4, with some returning after a 7- to 14-day treatment washout period (W) for the second treatment arm of the study. After 12 to 16 days of the end of the final arm, participants returned for a discharge interview (DC) before study termination.
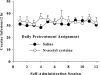
Figure 2
Daily _N_-acetyl cysteine does not alter the amount of cocaine self-administered by rats. Data depict the mean±SEM number of infusions across 12 maintenance self-administration sessions. Rats received _N_-acetyl cysteine (0 or 60 mg/kg, IP) 60 min before each session of cocaine self-administration (0.5 mg/kg, IV/infusion; 2 h/day; _N_=7/group).
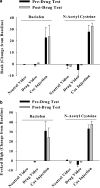
Figure 3
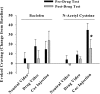
Daily _N_-acetyl cysteine does not alter cocaine-induced rush (a) or high (b) in cocaine-dependent human participants. Data depict a change in self-reports (mean±SEM) of rush and high from baseline values collected immediately before values obtained during exposure to a neutral video and cocaine video, or following a cocaine injection (20 mg/70 kg/60 s, IV). Measures of rush and high were obtained before and following daily baclofen (20 mg; TID; _N_=4) or _N_-acetyl cysteine (400 or 800 mg; TID; _N_=4) treatment.
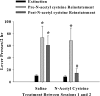
Figure 4
Daily _N_-acetyl cysteine decreases cocaine-induced reinstatement in rodents. Data depict the mean (±SEM) number of lever presses over during the last extinction test, or during two reinstatement tests. Rats self-administered cocaine (0.5 mg/kg/200 μl IV; 2-h/day; _N_=10–12/group) over 12 daily sessions. After 7 days of the last self-administration session, rats underwent daily extinction training in the absence of any drug treatment. This was followed by a test for cocaine-induced (10 mg/kg, IP) reinstatement. The day after the first reinstatement test, rats received seven daily injections of saline or _N_-acetyl cysteine (60 mg/kg, IP). The day after the seventh and final injection of saline or _N_-acetyl cysteine, rats underwent a second test for cocaine-induced reinstatement. #A significant difference from the first reinstatement test; _t_-test, p<0.05. *A significant difference from extinction responding, p<0.05.
Figure 5
_N_-acetyl cysteine reduces craving reported after a cocaine injection by human cocaine-dependent participants. Data depict a change in self-reports (mean±SEM) of craving from baseline values collected immediately before values obtained during exposure to a neutral video and cocaine video, or following a cocaine injection (20 mg/70 kg/60 s, IV). Measures of craving were obtained before and after daily baclofen (20 mg; TID; _N_=4) or _N_-acetyl cysteine (400 or 800 mg; TID; _N_=4) treatment. *A significant difference from craving reported following a cocaine injection on test day 1 (eg, pre-baclofen or _N_-acetyl cysteine treatment), p<0.05, _t_-test.
Similar articles
- Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine.
Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA. Madayag A, et al. J Neurosci. 2007 Dec 19;27(51):13968-76. doi: 10.1523/JNEUROSCI.2808-07.2007. J Neurosci. 2007. PMID: 18094234 Free PMC article. - Toward early estimation and treatment of addiction vulnerability: radial arm maze and N-acetyl cysteine before cocaine sensitization or nicotine self-administration in neonatal ventral hippocampal lesion rats.
Rao KN, Sentir AM, Engleman EA, Bell RL, Hulvershorn LA, Breier A, Chambers RA. Rao KN, et al. Psychopharmacology (Berl). 2016 Dec;233(23-24):3933-3945. doi: 10.1007/s00213-016-4421-8. Epub 2016 Sep 17. Psychopharmacology (Berl). 2016. PMID: 27640177 Free PMC article. - The effects of nicotinamide on reinstatement to cocaine seeking in male and female Sprague Dawley rats.
Witt EA, Reissner KJ. Witt EA, et al. Psychopharmacology (Berl). 2020 Mar;237(3):669-680. doi: 10.1007/s00213-019-05404-y. Epub 2019 Dec 7. Psychopharmacology (Berl). 2020. PMID: 31811351 Free PMC article. - Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction.
Schmidt HD, Pierce RC. Schmidt HD, et al. Ann N Y Acad Sci. 2010 Feb;1187:35-75. doi: 10.1111/j.1749-6632.2009.05144.x. Ann N Y Acad Sci. 2010. PMID: 20201846 Free PMC article. Review. - Kinase interest you in treating incubated cocaine-craving? A hypothetical model for treatment intervention during protracted withdrawal from cocaine.
Szumlinski KK, Shin CB. Szumlinski KK, et al. Genes Brain Behav. 2018 Mar;17(3):e12440. doi: 10.1111/gbb.12440. Epub 2017 Dec 20. Genes Brain Behav. 2018. PMID: 29152855 Free PMC article. Review.
Cited by
- A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence.
LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. LaRowe SD, et al. Am J Addict. 2013 Sep-Oct;22(5):443-52. doi: 10.1111/j.1521-0391.2013.12034.x. Epub 2013 May 15. Am J Addict. 2013. PMID: 23952889 Free PMC article. Clinical Trial. - Regulation of glutamate transporter 1 (GLT-1) gene expression by cocaine self-administration and withdrawal.
Kim R, Sepulveda-Orengo MT, Healey KL, Williams EA, Reissner KJ. Kim R, et al. Neuropharmacology. 2018 Jan;128:1-10. doi: 10.1016/j.neuropharm.2017.09.019. Epub 2017 Sep 14. Neuropharmacology. 2018. PMID: 28919080 Free PMC article. - Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens.
Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. Purgianto A, et al. Neuropsychopharmacology. 2013 Aug;38(9):1789-97. doi: 10.1038/npp.2013.78. Epub 2013 Apr 1. Neuropsychopharmacology. 2013. PMID: 23546386 Free PMC article. - Ameliorative Effects Of N-Acetylcysteine As Adjunct Therapy On Symptoms Of Painful Diabetic Neuropathy.
Heidari N, Sajedi F, Mohammadi Y, Mirjalili M, Mehrpooya M. Heidari N, et al. J Pain Res. 2019 Nov 19;12:3147-3159. doi: 10.2147/JPR.S228255. eCollection 2019. J Pain Res. 2019. PMID: 31819599 Free PMC article. - Evaluating N-acetylcysteine for early and end-of-treatment abstinence in adult cigarette smokers.
McClure EA, Wahlquist AE, Tomko RL, Baker NL, Carpenter MJ, Bradley ED, Cato PA, Gipson CD, Gray KM. McClure EA, et al. Drug Alcohol Depend. 2021 Aug 1;225:108815. doi: 10.1016/j.drugalcdep.2021.108815. Epub 2021 Jun 18. Drug Alcohol Depend. 2021. PMID: 34171822 Free PMC article. Clinical Trial.
References
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154:76–84. - PubMed
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. - PubMed
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, et al. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 DA019754-02/DA/NIDA NIH HHS/United States
- R01 DA015758/DA/NIDA NIH HHS/United States
- DA09465/DA/NIDA NIH HHS/United States
- R01 DA017328/DA/NIDA NIH HHS/United States
- Z01 DA000486/Intramural NIH HHS/United States
- R01 DA025617/DA/NIDA NIH HHS/United States
- R01 DA009465/DA/NIDA NIH HHS/United States
- F30 DA019754/DA/NIDA NIH HHS/United States
- R01 DA025617-02/DA/NIDA NIH HHS/United States
- RR00058/RR/NCRR NIH HHS/United States
- R01 DA010214-04/DA/NIDA NIH HHS/United States
- DA010214/DA/NIDA NIH HHS/United States
- K23 DA000486-02/DA/NIDA NIH HHS/United States
- R01 DA017328-04/DA/NIDA NIH HHS/United States
- M01 RR000058/RR/NCRR NIH HHS/United States
- DA025617/DA/NIDA NIH HHS/United States
- R01 DA015758-07/DA/NIDA NIH HHS/United States
- DA017328/DA/NIDA NIH HHS/United States
- DA015758/DA/NIDA NIH HHS/United States
- R01 DA010214/DA/NIDA NIH HHS/United States
- DA019754/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical