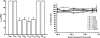Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? - PubMed (original) (raw)
Toxicity and cellular uptake of gold nanoparticles: what we have learned so far?
Alaaldin M Alkilany et al. J Nanopart Res. 2010 Sep.
Abstract
Gold nanoparticles have attracted enormous scientific and technological interest due to their ease of synthesis, chemical stability, and unique optical properties. Proof-of-concept studies demonstrate their biomedical applications in chemical sensing, biological imaging, drug delivery, and cancer treatment. Knowledge about their potential toxicity and health impact is essential before these nanomaterials can be used in real clinical settings. Furthermore, the underlying interactions of these nanomaterials with physiological fluids is a key feature of understanding their biological impact, and these interactions can perhaps be exploited to mitigate unwanted toxic effects. In this Perspective we discuss recent results that address the toxicity of gold nanoparticles both in vitro and in vivo, and we provide some experimental recommendations for future research at the interface of nanotechnology and biological systems.
Figures
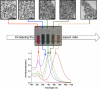
Fig. 1
Gold nanorods of different aspect ratios have different colors and tunable ultraviolet–visible–near-infrared spectra. Scale bars in the transmission electron micrographs at the top are 100 nm
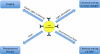
Fig. 2
Schematic showing the physical events that occur as a result of satisfying the localized surface plasmon resonance condition, with the corresponding applications. See text for details
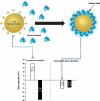
Fig. 3
(Upper panel): Cartoon demonstrating the formation of protein corona on a gold nanoparticle surface. Adsorption of serum proteins onto the surface of gold nanoparticles flips their effective surface charge. (Lower panel): Effective surface charge (zeta potential) of gold nanorods capped with cetyltrimethylammonium bromide, CTAB (white bars) and poly(acrylic acid), PAA (black bars). In aqueous solution, CTAB-capped gold nanorods have a positive effective surface charge and PAA-coated nanorods are negative. However, both have the same negative effective surface charge after they mixed with serum proteins and subsequently purified
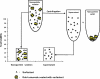
Fig. 4
“The supernatant control”. A gold nanorod solution is exposed to cells, and in this cartoon kills 70% of the cells at a certain dose. An identical gold nanorod solution is centrifuged, and the colorless supernatant exposed to cells. The similar toxicity of both solutions indicates that the nanoparticles are not toxic by themselves, but small molecules (leftover reagents, or desorbed capping agents) are
Fig. 5
Left: average lifespan of mice receiving gold nanoparticles, 8–37 nm in diameter, was shortened compared to smaller and larger nanoparticle sizes. The break marks on the top of bars indicate that no death was observed during the experimental period. Right: MTT assay for the same gold nanoparticles using the HeLa cell line. Images reproduced with permission from (Chen et al. 2009). Copyright: Springer Science
Fig. 6
Cartoon demonstrates the concept of the biodegradable plasmon-resonant liposomes. The whole composite absorbs in the near-infrared region and thus serve as “nanoheaters” to destroy cancer cells. Upon disruption of the carrier (liposomes), the nanoparticles could be released and have a higher chance to be bio-eliminated
Similar articles
- The gold standard: gold nanoparticle libraries to understand the nano-bio interface.
Alkilany AM, Lohse SE, Murphy CJ. Alkilany AM, et al. Acc Chem Res. 2013 Mar 19;46(3):650-61. doi: 10.1021/ar300015b. Epub 2012 Jun 25. Acc Chem Res. 2013. PMID: 22732239 - Plant-based gold nanoparticles; a comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications.
Khan T, Ullah N, Khan MA, Mashwani ZU, Nadhman A. Khan T, et al. Adv Colloid Interface Sci. 2019 Oct;272:102017. doi: 10.1016/j.cis.2019.102017. Epub 2019 Aug 8. Adv Colloid Interface Sci. 2019. PMID: 31437570 Review. - Recent biomedical applications of gold nanoparticles: A review.
Elahi N, Kamali M, Baghersad MH. Elahi N, et al. Talanta. 2018 Jul 1;184:537-556. doi: 10.1016/j.talanta.2018.02.088. Epub 2018 Feb 26. Talanta. 2018. PMID: 29674080 Review. - Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications.
Fadeel B, Garcia-Bennett AE. Fadeel B, et al. Adv Drug Deliv Rev. 2010 Mar 8;62(3):362-74. doi: 10.1016/j.addr.2009.11.008. Epub 2009 Nov 10. Adv Drug Deliv Rev. 2010. PMID: 19900497 Review. - Potential toxicity of engineered nanoparticles in mammalian germ cells and developing embryos: treatment strategies and anticipated applications of nanoparticles in gene delivery.
Das J, Choi YJ, Song H, Kim JH. Das J, et al. Hum Reprod Update. 2016 Sep;22(5):588-619. doi: 10.1093/humupd/dmw020. Epub 2016 Jul 6. Hum Reprod Update. 2016. PMID: 27385359 Review.
Cited by
- Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo.
Kotchey GP, Zhao Y, Kagan VE, Star A. Kotchey GP, et al. Adv Drug Deliv Rev. 2013 Dec;65(15):1921-32. doi: 10.1016/j.addr.2013.07.007. Epub 2013 Jul 12. Adv Drug Deliv Rev. 2013. PMID: 23856412 Free PMC article. Review. - Dual-wavelength photoacoustic atlas method to estimate fractional methylene blue and hemoglobin contents.
Gonzalez E, Lediju Bell M. Gonzalez E, et al. J Biomed Opt. 2022 Sep;27(9):096002. doi: 10.1117/1.JBO.27.9.096002. J Biomed Opt. 2022. PMID: 36050818 Free PMC article. - Citrate-Stabilized Gold Nanorods-Directed Osteogenic Differentiation of Multiple Cells.
Zhang Y, Li Y, Liao W, Peng W, Qin J, Chen D, Zheng L, Yan W, Li L, Guo Z, Wang P, Jiang Q. Zhang Y, et al. Int J Nanomedicine. 2021 Apr 12;16:2789-2801. doi: 10.2147/IJN.S299515. eCollection 2021. Int J Nanomedicine. 2021. PMID: 33880024 Free PMC article. - In vivo study of spherical gold nanoparticles: inflammatory effects and distribution in mice.
Chen H, Dorrigan A, Saad S, Hare DJ, Cortie MB, Valenzuela SM. Chen H, et al. PLoS One. 2013;8(2):e58208. doi: 10.1371/journal.pone.0058208. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469154 Free PMC article. - Synthesis of Mesoporous Silica Coated Gold Nanorods Loaded with Methylene Blue and Its Potentials in Antibacterial Applications.
Fernández-Lodeiro A, Djafari J, Fernández-Lodeiro J, Duarte MP, Muchagato Mauricio E, Capelo-Martínez JL, Lodeiro C. Fernández-Lodeiro A, et al. Nanomaterials (Basel). 2021 May 19;11(5):1338. doi: 10.3390/nano11051338. Nanomaterials (Basel). 2021. PMID: 34069626 Free PMC article.
References
- Alivisatos AP.Semiconductor clusters, nanocrystals, and quantum dots Science 1996271933–937.10.1126/science.271.5251.9331996Sci...271..933A - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources