Visual abnormalities associated with enhanced optic nerve myelination - PubMed (original) (raw)
Comparative Study
Visual abnormalities associated with enhanced optic nerve myelination
Minzhong Yu et al. Brain Res. 2011.
Abstract
Expression of the constitutively active serine/threonine kinase Akt in oligodendrocytes results in enhanced myelination in the CNS. Here, we have examined the effects of this Akt overexpression on optic nerve structure and on optic nerve function, assessed using the visual evoked potential (VEP). Transgenic mice have been generated with the Plp promoter driving expression of a modified form of Akt, in which aspartic acids are substituted for Thr308 and Ser473. These Plp-Akt-DD (Akt-DD) mice, and littermate controls, were studied at different ages. Optic nerves were examined anatomically at 2 and 6 months of age. At 2 months of age, optic nerves were substantially thicker in Akt-DD mice, reflecting an increase in myelination of optic nerve axons. By electron microscopy, myelin thickness was increased in Akt-DD optic nerve, with extended paranodal domains having excess paranodal loops, and the density of nodes of Ranvier was reduced, relative to control mice. We recorded VEPs in response to strobe flash ganzfeld stimuli presented after overnight dark- and light-adapted conditions at ages ranging from 1 to 10 months. It was possible to record a clear VEP from Akt-DD mice at all ages examined. At 1 month of age, VEP implicit times were somewhat shorter in Akt-DD transgenic mice than in control animals. Beyond 6months of age, VEP latencies were consistently delayed in Akt-DD transgenic mice. These abnormalities did not reflect an alteration in retinal function as there were no significant differences between ERGs obtained from control or Akt-DD transgenic mice. In young mice, the somewhat faster responses may reflect improved transmission due to increased myelination of optic nerve axons. In older mice, where the Akt-DD optic nerve is markedly thicker than control, it is remarkable that optic nerves continue to function.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Figure 1
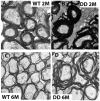
Representative electron micrographs of optic nerve cross-sections obtained from WT (A, C) or Akt-DD (B, D) mice at two (A, B) or 6 months of age (C, D). The myelin sheaths surrounding optic nerve axons are thicker in two-month old Akt-DD mice than in WT animals. The myelination process continues, and the myelin sheaths of six-month old Akt-DD mice are excessive and only loosely organized around some axons. Three animals were studied for each genotype. Magnification: 10,000×.
Figure 2
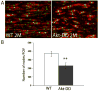
Density of nodes of Ranvier. (A) Representative images of nodes of Ranvier in optic nerve sections of WT (left) or Akt-DD (right) mice aged 2 months. Sections were stained using antibodies against sodium channel NaV1.6 to label nodes (green) and against Caspr (red) to label paranodes. (B) Quantification of the number of nodes in the optic nerve. Confocal microscope images (40×) were taken and the number of nodes having paranodes on both sides were quantified manually per field of view (FOV). There was a significant decrease (p<0.001) in the nodal density in Akt-DD mice, relative to WT. A minimum of three sections were measured per animal. Bars indicate average (± s.d.) results for three animals for each genotype.
Figure 3
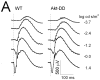
Representative ERGs obtained from representative 10-month old mice under dark-adapted (A) and light-adapted (B) conditions. Intensity-response functions for dark-adapted a- and b-wave (C) or light-adapted ERG (D). Data points indicate average (± s.d.) for 12 WT and 17 Akt-DD mice.
Figure 3
Representative ERGs obtained from representative 10-month old mice under dark-adapted (A) and light-adapted (B) conditions. Intensity-response functions for dark-adapted a- and b-wave (C) or light-adapted ERG (D). Data points indicate average (± s.d.) for 12 WT and 17 Akt-DD mice.
Figure 3
Representative ERGs obtained from representative 10-month old mice under dark-adapted (A) and light-adapted (B) conditions. Intensity-response functions for dark-adapted a- and b-wave (C) or light-adapted ERG (D). Data points indicate average (± s.d.) for 12 WT and 17 Akt-DD mice.
Figure 3
Representative ERGs obtained from representative 10-month old mice under dark-adapted (A) and light-adapted (B) conditions. Intensity-response functions for dark-adapted a- and b-wave (C) or light-adapted ERG (D). Data points indicate average (± s.d.) for 12 WT and 17 Akt-DD mice.
Figure 4
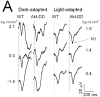
(A) Representative VEPs obtained from representative 8-month old WT and Akt-DD mice under dark-adapted (left) and light-adapted (right) conditions. (B-E) Intensity-response functions for N1 implicit times obtained under dark-adapted (B, D) or light-adapted (C,E) conditions from 1-month old (B, C) or 8-month old (D, E) mice. In (B, C), data points indicate average (± s.d.) for 30 WT and 19 Akt-DD mice tested at 1 month of age. In (D, E), data points indicate average (± s.d.) for 19 WT and 20 Akt-DD mice tested at 8 months of age. (F) Summary of age-related N1 implicit time changes in Akt-DD mice. Each data point indicates the difference between the average N1 implicit times for Akt-DD mice relative to that for WT mice. Dark-adapted data are represented by open symbols; light-adapted data are represented by filled symbols. Asterisks indicate ages where have significant differences in implicit times were noted between AKT and WT mice.
Figure 4
(A) Representative VEPs obtained from representative 8-month old WT and Akt-DD mice under dark-adapted (left) and light-adapted (right) conditions. (B-E) Intensity-response functions for N1 implicit times obtained under dark-adapted (B, D) or light-adapted (C,E) conditions from 1-month old (B, C) or 8-month old (D, E) mice. In (B, C), data points indicate average (± s.d.) for 30 WT and 19 Akt-DD mice tested at 1 month of age. In (D, E), data points indicate average (± s.d.) for 19 WT and 20 Akt-DD mice tested at 8 months of age. (F) Summary of age-related N1 implicit time changes in Akt-DD mice. Each data point indicates the difference between the average N1 implicit times for Akt-DD mice relative to that for WT mice. Dark-adapted data are represented by open symbols; light-adapted data are represented by filled symbols. Asterisks indicate ages where have significant differences in implicit times were noted between AKT and WT mice.
Figure 4
(A) Representative VEPs obtained from representative 8-month old WT and Akt-DD mice under dark-adapted (left) and light-adapted (right) conditions. (B-E) Intensity-response functions for N1 implicit times obtained under dark-adapted (B, D) or light-adapted (C,E) conditions from 1-month old (B, C) or 8-month old (D, E) mice. In (B, C), data points indicate average (± s.d.) for 30 WT and 19 Akt-DD mice tested at 1 month of age. In (D, E), data points indicate average (± s.d.) for 19 WT and 20 Akt-DD mice tested at 8 months of age. (F) Summary of age-related N1 implicit time changes in Akt-DD mice. Each data point indicates the difference between the average N1 implicit times for Akt-DD mice relative to that for WT mice. Dark-adapted data are represented by open symbols; light-adapted data are represented by filled symbols. Asterisks indicate ages where have significant differences in implicit times were noted between AKT and WT mice.
Figure 4
(A) Representative VEPs obtained from representative 8-month old WT and Akt-DD mice under dark-adapted (left) and light-adapted (right) conditions. (B-E) Intensity-response functions for N1 implicit times obtained under dark-adapted (B, D) or light-adapted (C,E) conditions from 1-month old (B, C) or 8-month old (D, E) mice. In (B, C), data points indicate average (± s.d.) for 30 WT and 19 Akt-DD mice tested at 1 month of age. In (D, E), data points indicate average (± s.d.) for 19 WT and 20 Akt-DD mice tested at 8 months of age. (F) Summary of age-related N1 implicit time changes in Akt-DD mice. Each data point indicates the difference between the average N1 implicit times for Akt-DD mice relative to that for WT mice. Dark-adapted data are represented by open symbols; light-adapted data are represented by filled symbols. Asterisks indicate ages where have significant differences in implicit times were noted between AKT and WT mice.
Figure 4
(A) Representative VEPs obtained from representative 8-month old WT and Akt-DD mice under dark-adapted (left) and light-adapted (right) conditions. (B-E) Intensity-response functions for N1 implicit times obtained under dark-adapted (B, D) or light-adapted (C,E) conditions from 1-month old (B, C) or 8-month old (D, E) mice. In (B, C), data points indicate average (± s.d.) for 30 WT and 19 Akt-DD mice tested at 1 month of age. In (D, E), data points indicate average (± s.d.) for 19 WT and 20 Akt-DD mice tested at 8 months of age. (F) Summary of age-related N1 implicit time changes in Akt-DD mice. Each data point indicates the difference between the average N1 implicit times for Akt-DD mice relative to that for WT mice. Dark-adapted data are represented by open symbols; light-adapted data are represented by filled symbols. Asterisks indicate ages where have significant differences in implicit times were noted between AKT and WT mice.
Figure 4
(A) Representative VEPs obtained from representative 8-month old WT and Akt-DD mice under dark-adapted (left) and light-adapted (right) conditions. (B-E) Intensity-response functions for N1 implicit times obtained under dark-adapted (B, D) or light-adapted (C,E) conditions from 1-month old (B, C) or 8-month old (D, E) mice. In (B, C), data points indicate average (± s.d.) for 30 WT and 19 Akt-DD mice tested at 1 month of age. In (D, E), data points indicate average (± s.d.) for 19 WT and 20 Akt-DD mice tested at 8 months of age. (F) Summary of age-related N1 implicit time changes in Akt-DD mice. Each data point indicates the difference between the average N1 implicit times for Akt-DD mice relative to that for WT mice. Dark-adapted data are represented by open symbols; light-adapted data are represented by filled symbols. Asterisks indicate ages where have significant differences in implicit times were noted between AKT and WT mice.
Similar articles
- Constitutively active Akt induces enhanced myelination in the CNS.
Flores AI, Narayanan SP, Morse EN, Shick HE, Yin X, Kidd G, Avila RL, Kirschner DA, Macklin WB. Flores AI, et al. J Neurosci. 2008 Jul 9;28(28):7174-83. doi: 10.1523/JNEUROSCI.0150-08.2008. J Neurosci. 2008. PMID: 18614687 Free PMC article. - Expression of a catalytically inactive transmembrane protein tyrosine phosphatase epsilon (tm-PTP epsilon) delays optic nerve myelination.
Muja N, Lovas G, Romm E, Machleder D, Ranjan M, Gallo V, Hudson LD. Muja N, et al. Glia. 2004 Dec;48(4):278-97. doi: 10.1002/glia.20078. Glia. 2004. PMID: 15390114 - Reversing hypomyelination in BACE1-null mice with Akt-DD overexpression.
Hu X, Schlanger R, He W, Macklin WB, Yan R. Hu X, et al. FASEB J. 2013 May;27(5):1868-73. doi: 10.1096/fj.12-224212. Epub 2013 Jan 18. FASEB J. 2013. PMID: 23335052 Free PMC article. - Dynamic Modulation of Myelination in Response to Visual Stimuli Alters Optic Nerve Conduction Velocity.
Etxeberria A, Hokanson KC, Dao DQ, Mayoral SR, Mei F, Redmond SA, Ullian EM, Chan JR. Etxeberria A, et al. J Neurosci. 2016 Jun 29;36(26):6937-48. doi: 10.1523/JNEUROSCI.0908-16.2016. J Neurosci. 2016. PMID: 27358452 Free PMC article. - Visually evoked potentials.
Creel DJ. Creel DJ. Handb Clin Neurol. 2019;160:501-522. doi: 10.1016/B978-0-444-64032-1.00034-5. Handb Clin Neurol. 2019. PMID: 31277872 Review.
Cited by
- Refinement of axonal conduction and myelination in the mouse optic nerve indicate an extended period of postnatal developmental plasticity.
Balraj A, Clarkson-Paredes C, Pajoohesh-Ganji A, Kay MW, Mendelowitz D, Miller RH. Balraj A, et al. Dev Neurobiol. 2022 May;82(4):308-325. doi: 10.1002/dneu.22875. Epub 2022 Apr 27. Dev Neurobiol. 2022. PMID: 35403346 Free PMC article. - Lutein and Zeaxanthin Isomers Reduce Photoreceptor Degeneration in the Pde6b rd10 Mouse Model of Retinitis Pigmentosa.
Yu M, Yan W, Beight C. Yu M, et al. Biomed Res Int. 2018 Dec 17;2018:4374087. doi: 10.1155/2018/4374087. eCollection 2018. Biomed Res Int. 2018. PMID: 30643804 Free PMC article. - The circadian clock gene Bmal1 is required to control the timing of retinal neurogenesis and lamination of Müller glia in the mouse retina.
Sawant OB, Jidigam VK, Fuller RD, Zucaro OF, Kpegba C, Yu M, Peachey NS, Rao S. Sawant OB, et al. FASEB J. 2019 Aug;33(8):8745-8758. doi: 10.1096/fj.201801832RR. Epub 2019 Apr 19. FASEB J. 2019. PMID: 31002540 Free PMC article. - Spontaneous optic nerve compression in the osteopetrotic (op/op) mouse: a novel model of myelination failure.
Kondo Y, Ramaker JM, Radcliff AB, Baldassari S, Mayer JA, Ver Hoeve JN, Zhang CL, Chiu SY, Colello RJ, Duncan ID. Kondo Y, et al. J Neurosci. 2013 Feb 20;33(8):3514-25. doi: 10.1523/JNEUROSCI.4849-12.2013. J Neurosci. 2013. PMID: 23426679 Free PMC article. - Carnosic acid slows photoreceptor degeneration in the Pde6b(rd10) mouse model of retinitis pigmentosa.
Kang K, Tarchick MJ, Yu X, Beight C, Bu P, Yu M. Kang K, et al. Sci Rep. 2016 Mar 10;6:22632. doi: 10.1038/srep22632. Sci Rep. 2016. PMID: 26961159 Free PMC article.
References
- Andrade EP, Sacai PY, Berezovsky A, Salomão SR. Pattern-reversal visual evoked potential abnormalities in patients with defined multiple sclerosis. Arq Bras Oftalmol. 2007;70:943–948. - PubMed
- Bjartmar C, Trapp BD. Axonal degeneration and progressive neurologic disability in multiple sclerosis. Neurotox Res. 2003;5:157–164. - PubMed
- Hu X, Hicks CW, He W, Wong P, Macklin WB, Trapp BD, Yan R. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9:1520–1525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS25304/NS/NINDS NIH HHS/United States
- R01 NS056417/NS/NINDS NIH HHS/United States
- R01 NS025304/NS/NINDS NIH HHS/United States
- R01NS56417/NS/NINDS NIH HHS/United States
- R24 EY015638/EY/NEI NIH HHS/United States
- R24EY15638/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials