Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2 - PubMed (original) (raw)
Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2
Hui Zheng et al. Mol Cell Biol. 2011 Feb.
Abstract
Alpha interferon (IFN-α) controls homeostasis of hematopoietic stem cells, regulates antiviral resistance, inhibits angiogenesis, and suppresses tumor growth. This cytokine is often used to treat cancers and chronic viral infections. The extent of cellular responses to IFN-α is limited by the IFN-induced ubiquitination and degradation of the IFN-α/β receptor chain 1 (IFNAR1) chain of the cognate receptor. IFNAR1 ubiquitination is facilitated by the βTrcp E3 ubiquitin ligase that is recruited to IFNAR1 upon its degron phosphorylation, which is induced by the ligand. Here we report identification of protein kinase D2 (PKD2) as a kinase that mediates the ligand-inducible phosphorylation of IFNAR1 degron and enables binding of βTrcp to the receptor. Treatment of cells with IFN-α induces catalytic activity of PKD2 and stimulates its interaction with IFNAR1. Expression and kinase activity of PKD2 are required for the ligand-inducible stimulation of IFNAR1 ubiquitination and endocytosis and for accelerated proteolytic turnover of IFNAR1. Furthermore, inhibition or knockdown of PKD2 robustly augments intracellular signaling induced by IFN-α and increases the efficacy of its antiviral effects. The mechanisms of the ligand-inducible elimination of IFNAR1 are discussed, along with the potential medical significance of this regulation.
Figures
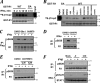
FIG. 1.
Pharmacological analyses of IFN-induced phosphorylation of IFNAR1 degron. (A) Binding of 35S-βTrcp2 to GST-IFNAR1 (wild type [WT] or S535 539A mutant [SA]) upon their phosphorylation using CK1α-depleted lysates from 293T cells treated with IFN-α as indicated. Levels of GST-IFNAR1 are analyzed by Coomassie blue staining. The SA mutant migrates more slowly in SDS-PAGE due to the presence of four additional amino acids in the linker (31). (B) The effects of various pharmacologic kinase inhibitors (whose activities were verified in kinase-specific assays; data not shown) on phosphorylation of GST-IFNAR1 by the CK1α-depleted lysate from IFN-α-treated (for 10 min) cells were analyzed by subsequent binding of 35S-labeled βTrcp2, as described for panel A. (C) Immunoblot analysis of Flag-IFNAR1 immunopurified from U3A human cells pretreated with kinase inhibitors and then treated with IFN-α as indicated. (D and E) Endogenous IFNAR1 immunoprecipitated from the lysates from 293T cells (treated as indicated) was analyzed as in panel C. (F) Immunoblot analysis of IFNAR1 and STAT1 in mouse embryo fibroblasts not treated (Mock) or treated with IFN-β (500 IU/ml) with or without pretreatment with JAK inhibitor I (JI) or PKD inhibitor CID755673 (CID) as indicated.
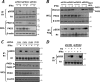
FIG. 2.
Ligand-induced phosphorylation of the IFNAR1 degron is mediated by PKD2. (A) Immunoblot analysis of endogenous IFNAR1 immunopurified from HeLa cells that received indicated siRNA oligonucleotides. Levels of PKD species and β-actin in whole-cell lysates (WCL) were also analyzed. (B) Immunoblot analysis of exogenous Flag-IFNAR1 immunopurified from U3A cells that received indicated siRNA oligonucleotides. Levels of PKD species and β-actin in WCL were also analyzed. WCL from 293T cells is used as a control of mobility of PKD2 species. (C) Immunoblot analysis of endogenous IFNAR1 immunopurified from 293T cells transduced with shRNA against GFP (CON) or PKD species (as indicated) and then treated with IFN-α was carried out as in panel A. Levels of PKD species and β-actin in WCL were also analyzed. (D) Immunoblot analysis of endogenous IFNAR1 immunopurified from HeLa cells stably transduced with shRNA against GFP (shCON) or PKD2 (shPKD2) and then treated with IFN-α or thapsigargin (TG).
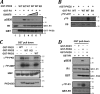
FIG. 3.
PKD2 is capable of phosphorylating the IFNAR1 degron in vitro. (A) Effect of Gö6976 (20 to 200 nM) on in vitro phosphorylation of GST-IFNAR1 on Ser535 by purified GST-PKD2 (wild type or kinase dead [KD]) was analyzed by immunoblotting. (B) In vitro phosphorylation of wild-type or SA mutant GST-IFNAR1 (96.0 pmol) by recombinant PKD2 (0.8 pmol) in the presence of γ-32P-labeled ATP was analyzed by autoradiography and Coomassie blue staining. (C) Indicated GST-tagged PKD proteins expressed in 293T cells and purified by pulldown with glutathione beads were incubated with myelin basic protein (MBP) and [γ-32P]ATP. Incorporation of labeled phosphate into PKD and MBP was analyzed by autoradiography. Levels of MBP (Coomassie blue staining) and GST-tagged PKD species (immunoblot with antibody against GST) are also shown. (D) In vitro phosphorylation of GST-IFNAR1 on Ser535 by purified GST-PKD species was carried out using either unlabeled (top two panels) or labeled (bottom two panels) ATP and analyzed by immunoblotting (as in panel A) or autoradiography (as in panel B).
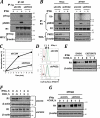
FIG. 4.
Ligand-induced recruitment of PKD2 to IFNAR1 and stimulation of PKD2 kinase activity. (A) IFNAR1 was immunoprecipitated from the lysates of 293T cells treated with IFN-α for the indicated times, and the reaction was analyzed by immunoblotting using the indicated antibodies. The leftmost lane of the upper panel represents the whole-cell lysates from untreated cells. (B) In vitro immunokinase activity of endogenous PKD2 purified from HeLa cells (treated with IFN-α as indicated) toward GST-IFNAR1 as a substrate in the presence of [γ-32P]ATP as analyzed by autoradiography (upper panel), CB staining (middle panel), and immunoblotting with an antibody against PKD (lower panel). (C) Immunoblot analysis of phosphorylation of Ser710 within the activation loop of PKD2 carried out on endogenous PKD2 immunoprecipitated from HeLa cells treated as indicated. (D) Immunoblot analysis on GST-PKD2 purified from HeLa cells was carried out as outlined for panel C. (E) Human 2fTGH fibrosarcoma cells or their isogenic Tyk2-deficient 11.1 derivatives were treated with IFN-α as indicated. Endogenous PKD2 was immunoprecipitated and analyzed as in panel C.
FIG. 5.
PKD2 regulates ubiquitination, endocytosis, and degradation of IFNAR1. (A) Immunoblot analysis of binding of βTrcp to IFNAR1 in HeLa cells (which received indicated shRNAs) analyzed by coimmunoprecipitation and immunoblotting with indicated antibodies. Ig, reaction with irrelevant isotype antibody control. Levels of βTrcp and β-actin in whole-cell lysates (WCL) were also analyzed. (B) Immunoblot analysis of IFNAR1 immunopurified from either HeLa or 2fTGH cells that received indicated shRNA. Levels of PKD2 and β-actin in whole-cell lysates (WCL) were also analyzed. (C) Effect of PKD2 knockdown (open squares) on the rate of internalization of endogenous IFNAR1 measured by a fluorescence-based assay are presented as percentages of total cell surface IFNAR1 level (means ± standard errors of the mean). (D) Fluorescence-activated cell sorter (FACS) analysis of IFNAR1 levels on the surface of cells that received indicated shRNA. Green, blue, and brown signals represent cell surface expression of IFNAR1 after 0, 1, and 2 h of IFN-α treatment, respectively (red, isotype control). (E) Immunoblot analysis of endogenous IFNAR1 in cells untreated or pretreated with the PKD inhibitor CID755673 and then subjected to a cycloheximide (CHX) chase in the presence of IFN-α for the indicated times. (F) Degradation of IFNAR1 in HeLa cells that received indicated shRNA was assessed as for panel E. (G) Degradation of IFNAR1 in 2fTGH cells that received indicated shRNA was assessed as for panel E.
FIG. 6.
PKD2 regulates the extent of cellular responses to IFN-α. (A) STAT1 Tyr phosphorylation and levels in cells untreated or pretreated with the PKD inhibitor CID755673 for 1 h and then pulse-treated with IFN-α for 15 min (followed by removal of cytokine and inhibitor and incubation of cells for the indicated times) were analyzed by immunoblotting. (B) Analysis in cells that received indicated shRNA was carried out as for panel A. (C) Relative activity of ISRE-driven firefly luciferase activity normalized to renilla luciferase activity in 2fTGH cells. Cells were pretreated with the PKD inhibitor CID755673 (for 1 h) and pulse-treated with IFN-α (for 0 to 2 h) as indicated, and the activity of luciferase was assessed 24 h later. Data from four independent experiments (each in triplicate) are presented as means ± standard errors of the mean. (D) Analysis of ISRE-driven transcription in 2fTGH cells that received the indicated shRNA and were pulse-treated with IFN-α for the indicated times was carried out as described for panel C. (E) Immunoblot analysis of PKR, STAT1, and β-actin levels in 2fTGH cells that received the indicated shRNA were pulse-treated with IFN-α for the indicated times and were analyzed 24 h thereafter.
FIG. 7.
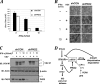
PKD2 regulates the antiviral defenses. (A) 2fTGH cells that received control shRNA (black bars) or shRNA against PKD2 (white bars) were untreated or pretreated with IFN-α (at the indicated doses) and then infected with VSV. Viral titers from three independent experiments (each in quadruplicate) are shown as means ± standard errors of the mean. (B) Cytopathogenic effect of VSV manifested in appearance of rounded, poorly attached, dying cells upon infection of 2fTGH cells that received indicated shRNAs and were pretreated with IFN-α as indicated and then infected with VSV (at an MOI of 0.5). (C) Viral load in 2fTGH cells transduced, treated, and infected with VSV as outlined for panel B was assessed by analysis of the expression of VSV-M viral protein by immunoblotting. Comparable loading was verified by analysis of β-actin (shown in the middle panel). The extent of PKD2 knockdown (lower panel) is also shown. (D) Model that outlines pathways that converge on the phosphorylation of the IFNAR1 degron and ensuing βTrcp-dependent ubiquitination and degradation of IFNAR1 and restriction of cellular responses to type I IFN. Whereas the ligand-independent “inside-out” pathway triggered by unfolded protein responses (UPR) relies on activity of PERK (2, 31) and phosphorylation of the IFNAR1 degron by CK1α (30), the IFN-α/β-inducible pathway involves JAK-mediated activation of PKD2.
Similar articles
- Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor.
Bhattacharya S, Qian J, Tzimas C, Baker DP, Koumenis C, Diehl JA, Fuchs SY. Bhattacharya S, et al. J Biol Chem. 2011 Jun 24;286(25):22069-76. doi: 10.1074/jbc.M111.238766. Epub 2011 May 3. J Biol Chem. 2011. PMID: 21540188 Free PMC article. - Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor.
Bhattacharya S, HuangFu WC, Liu J, Veeranki S, Baker DP, Koumenis C, Diehl JA, Fuchs SY. Bhattacharya S, et al. J Biol Chem. 2010 Jan 22;285(4):2318-25. doi: 10.1074/jbc.M109.071498. Epub 2009 Nov 30. J Biol Chem. 2010. PMID: 19948722 Free PMC article. - Tyrosine phosphorylation of protein kinase D2 mediates ligand-inducible elimination of the Type 1 interferon receptor.
Zheng H, Qian J, Baker DP, Fuchs SY. Zheng H, et al. J Biol Chem. 2011 Oct 14;286(41):35733-35741. doi: 10.1074/jbc.M111.263608. Epub 2011 Aug 24. J Biol Chem. 2011. PMID: 21865166 Free PMC article. - Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis.
Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Zheng H, et al. Blood. 2011 Oct 6;118(14):4003-6. doi: 10.1182/blood-2011-06-359745. Epub 2011 Aug 10. Blood. 2011. PMID: 21832278 Free PMC article. - Ligand-independent pathway that controls stability of interferon alpha receptor.
Liu J, Plotnikov A, Banerjee A, Suresh Kumar KG, Ragimbeau J, Marijanovic Z, Baker DP, Pellegrini S, Fuchs SY. Liu J, et al. Biochem Biophys Res Commun. 2008 Mar 7;367(2):388-93. doi: 10.1016/j.bbrc.2007.12.137. Epub 2007 Dec 31. Biochem Biophys Res Commun. 2008. PMID: 18166147 Free PMC article.
Cited by
- Virus versus host: influenza A virus circumvents the immune responses.
Su G, Chen Y, Li X, Shao JW. Su G, et al. Front Microbiol. 2024 May 16;15:1394510. doi: 10.3389/fmicb.2024.1394510. eCollection 2024. Front Microbiol. 2024. PMID: 38817972 Free PMC article. Review. - PARP1 Enhances Influenza A Virus Propagation by Facilitating Degradation of Host Type I Interferon Receptor.
Xia C, Wolf JJ, Sun C, Xu M, Studstill CJ, Chen J, Ngo H, Zhu H, Hahm B. Xia C, et al. J Virol. 2020 Mar 17;94(7):e01572-19. doi: 10.1128/JVI.01572-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31915279 Free PMC article. - Context Is Key: Delineating the Unique Functions of IFNα and IFNβ in Disease.
Fox LE, Locke MC, Lenschow DJ. Fox LE, et al. Front Immunol. 2020 Dec 21;11:606874. doi: 10.3389/fimmu.2020.606874. eCollection 2020. Front Immunol. 2020. PMID: 33408718 Free PMC article. Review. - Ubiquitin-dependent Turnover of Adenosine Deaminase Acting on RNA 1 (ADAR1) Is Required for Efficient Antiviral Activity of Type I Interferon.
Li L, Qian G, Zuo Y, Yuan Y, Cheng Q, Guo T, Liu J, Liu C, Zhang L, Zheng H. Li L, et al. J Biol Chem. 2016 Nov 25;291(48):24974-24985. doi: 10.1074/jbc.M116.737098. Epub 2016 Oct 11. J Biol Chem. 2016. PMID: 27729454 Free PMC article. - Protein tyrosine phosphatase 1B is a key regulator of IFNAR1 endocytosis and a target for antiviral therapies.
Carbone CJ, Zheng H, Bhattacharya S, Lewis JR, Reiter AM, Henthorn P, Zhang ZY, Baker DP, Ukkiramapandian R, Bence KK, Fuchs SY. Carbone CJ, et al. Proc Natl Acad Sci U S A. 2012 Nov 20;109(47):19226-31. doi: 10.1073/pnas.1211491109. Epub 2012 Nov 5. Proc Natl Acad Sci U S A. 2012. PMID: 23129613 Free PMC article.
References
- Aaronson, D. S., and C. M. Horvath. 2002. A road map for those who don't know JAK-STAT. Science 296:1653-1655. - PubMed
- Coccia, E. M., G. Uze, and S. Pellegrini. 2006. Negative regulation of type I interferon signaling: facts and mechanisms. Cell. Mol. Biol. (Noisy-le-grand) 52:77-87. - PubMed
- Davis, G. L. 2001. Current treatment for chronic hepatitis C. Rev. Gastroenterol. Disord. 1:59-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous