Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse - PubMed (original) (raw)
Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse
Khaled Moussawi et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Cocaine addiction remains without an effective pharmacotherapy and is characterized by an inability of addicts to inhibit relapse to drug use. Vulnerability to relapse arises from an enduring impairment in cognitive control of motivated behavior, manifested in part by dysregulated synaptic potentiation and extracellular glutamate homeostasis in the projection from the prefrontal cortex to the nucleus accumbens. Here we show in rats trained to self-administer cocaine that the enduring cocaine-induced changes in synaptic potentiation and glutamate homeostasis are mechanistically linked through group II metabotropic glutamate receptor signaling. The enduring cocaine-induced changes in measures of cortico-accumbens synaptic and glial transmission were restored to predrug parameters for at least 2 wk after discontinuing chronic treatment with the cystine prodrug, N-acetylcysteine. N-acetylcysteine produced these changes by inducing an enduring restoration of nonsynaptic glutamatergic tone onto metabotropic glutamate receptors. The long-lasting pharmacological restoration of cocaine-induced glutamatergic adaptations by chronic N-acetylcysteine also caused enduring inhibition of cocaine-seeking in an animal model of relapse. These data mechanistically link nonsynaptic glutamate to cocaine-induced adaptations in excitatory transmission and demonstrate a mechanism to chronically restore prefrontal to accumbens transmission and thereby inhibit relapse in an animal model.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
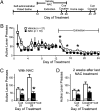
Fig. 1.
_N_-acetylcysteine (NAC) treatment during extinction training produced long-lasting protection from cue-induced and cocaine- and cue-induced reinstatement of lever pressing. (A) Treatment protocol for cocaine and NAC. (B) Active lever pressing during self-administration and extinction sessions. A two-way ANOVA over extinction (days 13–24) revealed a significant interaction between treatment group and time [F(11, 407) = 3.08, P < 0.001]. *P < 0.05, comparing vehicle to NAC using a Bonferroni multiple comparisons test. (C and D) Reinstatement of active lever pressing by cues (tone and light) or cocaine and cues (10 mg/kg, i.p.) was significantly inhibited both in the presence of NAC (days 25 and 29) (C) and 2–3 wk after discontinuing NAC treatment (days 43 and 47) (D). Student's t tests revealed Cue25: t(37) = 2.67, P = 0.011; Cocaine29: t(37) = 4.12, P < 0.001; Cue43: t(37) = 3.13, P = 0.003; Cocaine47: t(37) = 3.14, P = 0.003. *P < 0.05 comparing NAC to saline-treated groups.
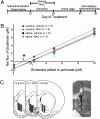
Fig. 2.
No-net-flux microdialysis in the NAcore reveals that chronic _N_-acetylcysteine (NAC) restores the reduced levels of extracellular glutamate elicited by cocaine self-administration. (A) Treatment protocol for cocaine and NAC used for data shown in Figs. 2, 3 and 5. (B) Plots from the no-net-flux in vivo microdialysis experiment. Net flux (y axis) is the difference between the glutamate concentration dialyzed into and recovered from the brain. The x-intercept corresponding to the extracellular glutamate concentration was extrapolated from fitted regression lines and differed between treatment groups: cocaine, saline 1.16 ± 0.17 μM; cocaine, NAC 1.98 ± 0.22 μM; saline, saline 2.19 ± 0.63 μM; saline, NAC 2.09 ± 0.61 μM [F(3, 115) = 3.90; P = 0.011]. There was no difference in the slope of the regression line between the four groups. *P < 0.05 for (cocaine, saline) compared with the other three groups using a Bonferroni test for multiple comparisons. (C) Summary and micrograph example of the location of the active membrane of the dialysis probe in the NAcore according to Paxinos and Watson (47). ac, anterior commissure; arrows, dorsal and ventral extent of the active dialysis membrane.
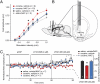
Fig. 3.
_N_-acetylcysteine (NAC) restores synaptic strength in prefrontal cortex projections to the NAcore through modulating mGluR2/3 receptors. (A) Input–output curve measured in the NAcore following in vivo stimulation of the prefrontal cortex. The increase in field amplitude in rats with a history of cocaine self-administration was reduced by NAC treatment during extinction training; two-way ANOVA [treatment group F(3, 150) = 3.83; P = 0.022; stimulation intensity F(6, 18) = 409.3; P < 0.001; interaction F(18, 150) = 1.77; P = 0.034]. *P < 0.05, for comparing cocaine, saline to other groups using a Bonferroni post hoc test. (B) Illustration of the experimental preparation where a dialysis probe and adjacent recording electrode were implanted into the NAcore. (C) Field potentials (normalized to levels following aCSF) were increased in yoked-saline control and NAC, but not vehicle-treated animals trained to self-administer cocaine after two concentrations of the mGluR2/3 antagonist LY341495 were delivered through a dialysis probe. Two-way repeated-measures ANOVA revealed significant group and time effects of LY341495 [group: F (2, 493) = 5.07, P = 0.019; time: F(29, 493) = 1.59, P = 0.027]. (Inset) Mean ± SEM normalized field amplitude averaged over the last 10 min (last 5 data samples were averaged) after 20 μm LY341495 [one-way ANOVA: F(2, 19) = 7.04, P = 0.006]. *P < 0.05 for (cocaine, saline) compared with other groups using a Bonferroni multiple comparisons test.
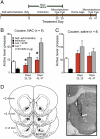
Fig. 4.
Enduring protection from relapse to cocaine-seeking by _N_-acetylcysteine is mGluR2/3-dependent. (A) Treatment protocol for data in B. (B) The inhibition of reinstated cocaine-seeking in the _N_-acetylcysteine treatment group was reversed by intra-NAcore microinjection of LY341495; paired Student's t test: days 25, 29 [t(7) = 2.57, P = 0.037]; days 43, 47 [t(7) = 3.92, P = 0.006]. (C) LY341495 did not alter reinstatement in the saline treatment group. (D) Illustration and example micrograph showing the location of microinjection cannula tips in the NAcore. ac, anterior commissure; arrow, injection site in the dorsomedial NAcore. *P < 0.05, comparing LY341495 to aCSF.
Fig. 5.
_N_-acetylcysteine (NAC) restores mEPSC frequency and the AMPA:NMDA ratio. (A) Example of mEPSC from each treatment group. Calibration: 500 msec; 25.6 pA. (B) Cumulative probability and mean values showing mEPSC frequency is increased by cocaine self-administration and was normalized by NAC. n = no. of cells, no. of animals; one-way ANOVA: F(2, 27) = 6.61, P = 0.005; *P < 0.05, compared with saline, vehicle. (C) Cumulative probability and mean values indicating mEPSC amplitude did not differ between treatment groups. (D) (Upper) Sample EPSC from the three different treatment groups illustrating total-, AMPA-, and NMDA-mediated currents. Calibration: 100 ms; 100 pA. (Lower) The increase in AMPA:NMDA ratio after withdrawal from self-administered cocaine is normalized to control values by NAC. One-way ANOVA: F(2, 40) = 6.57, P = 0.004. *P < 0.05 for (cocaine, saline) compared with other groups using a Bonferroni multiple comparisons test.
Similar articles
- The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine.
Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. Kupchik YM, et al. Biol Psychiatry. 2012 Jun 1;71(11):978-86. doi: 10.1016/j.biopsych.2011.10.024. Epub 2011 Dec 3. Biol Psychiatry. 2012. PMID: 22137594 Free PMC article. - Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking.
Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Moran MM, et al. J Neurosci. 2005 Jul 6;25(27):6389-93. doi: 10.1523/JNEUROSCI.1007-05.2005. J Neurosci. 2005. PMID: 16000629 Free PMC article. - N-Acetylcysteine reverses cocaine-induced metaplasticity.
Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. Moussawi K, et al. Nat Neurosci. 2009 Feb;12(2):182-9. doi: 10.1038/nn.2250. Epub 2009 Jan 11. Nat Neurosci. 2009. PMID: 19136971 Free PMC article. - Glutamate: the new frontier in pharmacotherapy for cocaine addiction.
Uys JD, LaLumiere RT. Uys JD, et al. CNS Neurol Disord Drug Targets. 2008 Nov;7(5):482-91. doi: 10.2174/187152708786927868. CNS Neurol Disord Drug Targets. 2008. PMID: 19128205 Review. - Metabotropic glutamate 7 (mGlu7) receptor: a target for medication development for the treatment of cocaine dependence.
Li X, Xi ZX, Markou A. Li X, et al. Neuropharmacology. 2013 Mar;66:12-23. doi: 10.1016/j.neuropharm.2012.04.010. Epub 2012 Apr 21. Neuropharmacology. 2013. PMID: 22546614 Free PMC article. Review.
Cited by
- Reduction in phencyclidine induced sensorimotor gating deficits in the rat following increased system xc⁻ activity in the medial prefrontal cortex.
Lutgen V, Qualmann K, Resch J, Kong L, Choi S, Baker DA. Lutgen V, et al. Psychopharmacology (Berl). 2013 Apr;226(3):531-40. doi: 10.1007/s00213-012-2926-3. Epub 2012 Nov 29. Psychopharmacology (Berl). 2013. PMID: 23192314 Free PMC article. - N-Acetyl-cysteine causes analgesia by reinforcing the endogenous activation of type-2 metabotropic glutamate receptors.
Bernabucci M, Notartomaso S, Zappulla C, Fazio F, Cannella M, Motolese M, Battaglia G, Bruno V, Gradini R, Nicoletti F. Bernabucci M, et al. Mol Pain. 2012 Oct 23;8:77. doi: 10.1186/1744-8069-8-77. Mol Pain. 2012. PMID: 23088864 Free PMC article. - Extracellular glutamate: functional compartments operate in different concentration ranges.
Moussawi K, Riegel A, Nair S, Kalivas PW. Moussawi K, et al. Front Syst Neurosci. 2011 Nov 24;5:94. doi: 10.3389/fnsys.2011.00094. eCollection 2011. Front Syst Neurosci. 2011. PMID: 22275885 Free PMC article. - The neurobiology of addiction.
Uhl GR, Koob GF, Cable J. Uhl GR, et al. Ann N Y Acad Sci. 2019 Sep;1451(1):5-28. doi: 10.1111/nyas.13989. Epub 2019 Jan 15. Ann N Y Acad Sci. 2019. PMID: 30644552 Free PMC article. - Effects of Methamphetamine Self-Administration and Extinction on Astrocyte Structure and Function in the Nucleus Accumbens Core.
Siemsen BM, Reichel CM, Leong KC, Garcia-Keller C, Gipson CD, Spencer S, McFaddin JA, Hooker KN, Kalivas PW, Scofield MD. Siemsen BM, et al. Neuroscience. 2019 May 15;406:528-541. doi: 10.1016/j.neuroscience.2019.03.040. Epub 2019 Mar 26. Neuroscience. 2019. PMID: 30926546 Free PMC article.
References
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. - PubMed
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. - PubMed
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–387. - PubMed
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. - PubMed
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA012513/DA/NIDA NIH HHS/United States
- T32007288/PHS HHS/United States
- P50 DA015369/DA/NIDA NIH HHS/United States
- R01 DA003906/DA/NIDA NIH HHS/United States
- C06 RR015455/RR/NCRR NIH HHS/United States
- RR015455/RR/NCRR NIH HHS/United States
- R37 DA003906/DA/NIDA NIH HHS/United States
- DA015369/DA/NIDA NIH HHS/United States
- DA003906/DA/NIDA NIH HHS/United States
- R01 DA012513/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical