Lithium rescues the impaired autophagy process in CbCln3(Δex7/8/Δex7/8) cerebellar cells and reduces neuronal vulnerability to cell death via IMPase inhibition - PubMed (original) (raw)
Comparative Study
Lithium rescues the impaired autophagy process in CbCln3(Δex7/8/Δex7/8) cerebellar cells and reduces neuronal vulnerability to cell death via IMPase inhibition
Jae-Woong Chang et al. J Neurochem. 2011 Feb.
Abstract
Juvenile neuronal ceroid lipofuscinosis (Batten disease) is a neurodegenerative disorder caused by mutation in CLN3. Defective autophagy and concomitant accumulation of autofluorescence enriched with mitochondrial ATP synthase subunit c were previously discovered in Cln3 mutant knock-in mice. In this study, we show that treatment with lithium reduces numbers of LC3-positive autophagosomes and accumulation of LC3-II in Cln3 mutant knock-in cerebellar cells (CbCln3(Δex7/8/Δex7/8) ). Lithium, an inhibitor of GSK3 and IMPase, reduces the accumulation of mitochondrial ATP synthase subunit c and autofluorescence in CbCln3(Δex7/8/Δex7/8) cells, and mitigates the abnormal subcellular distribution of acidic vesicles in the cells. L690,330, an IMPase inhibitor, is as effective as lithium in restoring autophagy in CbCln3(Δex7/8/Δex7/8) cells. Moreover, lithium or down-regulation of IMPase expression protects CbCln3(Δex7/8/Δex7/8) cells from cell death induced by amino acid deprivation. These results suggest that lithium overcomes the autophagic defect in CbCln3(Δex7/8/Δex7/8) cerebellar cells probably through IMPase, thereby reducing their vulnerability to cell death.
© 2011 The Authors. Journal of Neurochemistry © 2011 International Society for Neurochemistry.
Figures
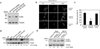
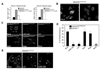
Fig. 1. Conversion of LC3-I to LC3-II is enhanced by CLN3 deficiency
(A) Generation of CLN3 knockdown cells. SH-SY5Y cells were stably transfected with control pcDNA (SH-SY5Y pcDNA), p_CLN3_ AS (SH-SY5Y CLN3 AS; anti-sense cDNA of CLN3) or p_CLN3_ (SH-SY5Y CLN3), after which CLN3 expression was assessed by Western blotting with anti-CLN3 antibody. (B and C) Defective autophagic maturation in CLN3 knockdown cells. SH-SY5Y pcDNA, SH-SY5Y CLN3 or SH-SY5Y CLN3 AS cells were transiently transfected with mCherry-GFP-LC3 for 48 h and then examined under a confocal microscope. Arrows indicate cellular colocalization of mCherry and GFP (B). The numbers of cells showing only red fluorescence in B were quantified and are represented as bars (means ± S.D., n > 30 cells). Asterisk indicates a significant difference from control (p < 0.05) (C). (D) CLN3 deletion increases LC3-II levels. Whole brains extracts from Cln3 knock-out (K/O) mice and age-matched control (WT) mice, cell extracts from SH-SY5Y pcDNA and SH-SY5Y CLN3 AS cells, lymphoblastoid cell extracts of JNCL patients (DT5) and age-matched controls (3798), and cerebellar cell extracts from Cb_Cln3_+/+ (wild-type) and Cb_Cln3_Δex7/8/Δex7/8 (homozygous Cb_Cln3_Δex7/8) mice were analyzed by Western blotting using anti-LC3 and anti-Tubulin antibodies. LC3-II levels on the blot were quantified by densitometric analysis and normalized by those of Tubulin. Their relative ratios to the control (WT) are represented (LC3-II level). (E) Defective autophagic maturation in Cln3 knock-in cerebellar cells. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were left untreated (Mock) or incubated with 20 nM bafilomycin A1 (Baf A1) for 2 h. Cell extracts were then prepared and analyzed by Western blotting using anti-LC3 antibody. The LC3-II signals on the blot were quantified as described in D.
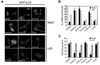
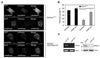
Fig. 2. Lithium reduces the accumulation of LC3 dots resulting from CLN3 mutation
(A) Photomicrograph showing the effect of CLN3 deletion on LC3 dot formation. SH-SY5Y-LC3 cells were transiently transfected with pcDNA, p_CLN3_Δ1 (1– 153), p_CLN3_Δ2 (1–263), p_CLN3_Δ3 (154–438), p_CLN_3 AS or p_CLN_3 for 24 h and then examined for LC3 dot formation. (B) Inhibition of LC3 dot formation by lithium. SH-SY5Y-LC3 cells were transiently transfected as in (A) and then treated with 10 mM NaCl or 10 mM LiCl for 24 h. The formation of GFP-LC3-positive dots was assessed using a fluorescence microscope. Bars indicate means ± S.D. (n > 30 cells). Asterisks indicate significant difference from control (p < 0.05). (C) SH-SY5Y cells were transiently cotransfected with mCherry-GFP-LC3 and either pcDNA, p_CLN3_Δ1 (1–153), p_CLN3_Δ2 (1–263), p_CLN3_Δ3 (154–438), p_CLN_3 AS or p_CLN_3 for 24 h and then left untreated (Mock) or treated with 10 mM LiCl for 24 h. The numbers of cells showing only red fluorescence were counted under a confocal microscope as described in Materials and methods. Bars indicate means ± S.D. (_n_ > 30 cells). Asterisks indicate significant difference from control (p < 0.05)
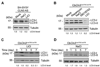
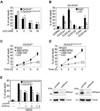
Fig. 3. Lithium reduces LC3-II level in SH-SY5Y CLN3 knockdown and Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells
(A) SH-SY5Y CLN3 AS cells were left untreated (Mock) or treated for 7 days with 10 mM NaCl (control) or 10 mM LiCl. Cell extracts were then prepared and analyzed by Western blotting with anti-LC3 antibody. (B) Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were left untreated (Mock) or treated for 7 days with 0.2 µM rapamycin, 10 mM NaCl (control), 10 µM SB216763 (GSK inhibitor) or 10 mM LiCl and then analyzed as in (A). (C and D) Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were treated with 10 mM LiCl (C) or 10 mM NaCl (D) for the indicated times and analyzed as in (A). The LC3-II signals on the blot were quantified as described in Fig. 1D.
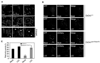
Fig. 4. Lithium promotes perinuclear localization of acidic vesicles and autophagic maturation in Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells
(A) Effect of lithium on the subcellular distribution of lysosomes. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were treated for 48 h with 10 mM NaCl, 10 µM SB216763 or 10 mM LiCl, incubated with Lysotracker (red), and then examined under a fluorescence microscope. “N” indicates the nucleus. (B and C) Effect of lithium on autophagic maturation. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were left untreated (Mock) or pretreated with 10 mM LiCl. After 24 h, the cells were transfected with mCherry-GFPLC3 for 48 h in the absence or presence of 10 mM LiCl and then visualized under a confocal microscope (B). The numbers of cells showing only red fluorescence in B were counted as described in Fig. 2B (C).
Fig. 5. Lithium or an IMPase inhibitor reduces accumulation of mitochondrial ATP synthase subunit c and autofluorescence in Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells
(A and B) Effect of lithium on the deposition of mitochondrial ATP synthase subunit c. Cb_Cln3_+/+ or Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were left untreated or incubated with 10 mM LiCl for 14 days and then immunostained using anti-mitochondrial ATP synthase subunit c antibody. The numbers of vesicles per cell (mean vesicle count/cell) were counted under a fluorescence microscope (A) and a typical image is shown (B). (C and D) Effect of lithium on the accumulation of autofluorescence. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were left untreated (Mock) or incubated with 10 mM NaCl, 100 µM L690,330, 0.2 µM rapamycin (Rapa) or 10 mM LiCl for 7 days. The autofluorescence (arrows) was visualized under a confocal microscope (C). Cells showing autofluorescence were counted and their percentage among total cell numbers are represented as bars (means ± S.D., n > 30). Asterisk indicates significant difference from control (p < 0.05) (D). (E) Effect of L690,330 on the subcellular distribution of lysosomes. Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were left untreated (Mock) or treated with 10 mM LiCl or 100 µM L690,330 for 48 h, incubated with Lysotracker (red) and then examined under a fluorescence microscope. “N” indicates the nucleus.
Fig. 6. Knockdown of IMPase1 increases autophagic maturation in Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells
(A–C) Effect of IMPase knockdown on autophagic maturation in Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cells were transiently co-transfected with mCherry-GFP-LC3 and either control shRNA (pSUPER) or IMPase1 shRNA (shIMPase1) for 48 h. Cells were then examined under a confocal microscope (A) and the numbers of cells showing only red fluorescence in A were counted (B). Bars indicate means ± S.D. (n > 20 cells) with significant difference from control (pSUPER) (p < 0.05). Expression level of IMPase1 was examined by quantitative RT-PCR (left) and Western blotting using IMPase1 antibody (right) (C).
Fig. 7. Lithium reduces the vulnerability of Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells to starvation-induced cell death
(A) Effect of lithium on the sensitivity of Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells to starvation-induced cell death. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cerebellar cells were incubated in amino acid-free medium for 12 h in the absence or presence of LiCl, after which the incidences of cell death were determined using a Live/Dead cell assay. Bars indicate means ± S.D. (n > 3). (B) Suppression of CLN3 mutation-induced cell death by lithium. SH-SY5Y cells were transiently transfected with pcDNA, p_CLN3_Δ1, p_CLN3_Δ2, p_CLN3_Δ3, p_CLN3_ AS or p_CLN3_, after which cells were treated for 24 h with 10 mM NaCl or 10 mM LiCl. Cell death assays were performed as in (A). (C and D) Suppression of amino aciddeprivation- induced cell death by L390,330 IMPase inhibitor. Cb_Cln3_+/+ (C) and Cb_Cln3_Δex7/8/Δex7/8 (D) cerebellar cells were incubated for the indicated times in amino acid-free medium in the absence or presence of 10 mM NaCl, 10 µM SB216763, 10 µM L690,330 or 10 mM LiCl. Cell death assays were performed as in (A) and values indicate means ± S.D. (n = 3). (E) Reducing IMPase expression suppresses amino acid deprivation-induced cell death. Cb_Cln3_+/+ and Cb_Cln3_Δex7/8/Δex7/8 cells were transiently transfected for 48 h with IMPase1 shRNA, IMPase2 shRNA or both, after which they were incubated for 9 h with complete medium or amino acid-free medium (HBSS). Cell death assays were performed as in (A) and values indicate means ± S.D. (n = 3) (left). Expression levels of IMPase1 and IMPase2 were determined by Western blotting using IMPase1 and IMPase2 antibodies (right). Asterisks indicate significant difference from control (p < 0.05).
Similar articles
- Distinct early molecular responses to mutations causing vLINCL and JNCL presage ATP synthase subunit C accumulation in cerebellar cells.
Cao Y, Staropoli JF, Biswas S, Espinola JA, MacDonald ME, Lee JM, Cotman SL. Cao Y, et al. PLoS One. 2011 Feb 17;6(2):e17118. doi: 10.1371/journal.pone.0017118. PLoS One. 2011. PMID: 21359198 Free PMC article. - Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis.
Cao Y, Espinola JA, Fossale E, Massey AC, Cuervo AM, MacDonald ME, Cotman SL. Cao Y, et al. J Biol Chem. 2006 Jul 21;281(29):20483-93. doi: 10.1074/jbc.M602180200. Epub 2006 May 19. J Biol Chem. 2006. PMID: 16714284 - Altered sensitivity of cerebellar granule cells to glutamate receptor overactivation in the Cln3(Δex7/8)-knock-in mouse model of juvenile neuronal ceroid lipofuscinosis.
Finn R, Kovács AD, Pearce DA. Finn R, et al. Neurochem Int. 2011 May;58(6):648-55. doi: 10.1016/j.neuint.2011.02.003. Epub 2011 Feb 17. Neurochem Int. 2011. PMID: 21315126 Free PMC article. - Microglia in juvenile neuronal ceroid lipofuscinosis are primed toward a pro-inflammatory phenotype.
Xiong J, Kielian T. Xiong J, et al. J Neurochem. 2013 Oct;127(2):245-58. doi: 10.1111/jnc.12385. Epub 2013 Aug 22. J Neurochem. 2013. PMID: 23919525 - Partial correction of the CNS lysosomal storage defect in a mouse model of juvenile neuronal ceroid lipofuscinosis by neonatal CNS administration of an adeno-associated virus serotype rh.10 vector expressing the human CLN3 gene.
Sondhi D, Scott EC, Chen A, Hackett NR, Wong AM, Kubiak A, Nelvagal HR, Pearse Y, Cotman SL, Cooper JD, Crystal RG. Sondhi D, et al. Hum Gene Ther. 2014 Mar;25(3):223-39. doi: 10.1089/hum.2012.253. Epub 2014 Mar 4. Hum Gene Ther. 2014. PMID: 24372003 Free PMC article.
Cited by
- Autophagy in the Neuronal Ceroid Lipofuscinoses (Batten Disease).
Kim WD, Wilson-Smillie MLDM, Thanabalasingam A, Lefrancois S, Cotman SL, Huber RJ. Kim WD, et al. Front Cell Dev Biol. 2022 Feb 16;10:812728. doi: 10.3389/fcell.2022.812728. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252181 Free PMC article. Review. - Progress in the Development of Small Molecule Therapeutics for the Treatment of Neuronal Ceroid Lipofuscinoses (NCLs).
Kinarivala N, Trippier PC. Kinarivala N, et al. J Med Chem. 2016 May 26;59(10):4415-27. doi: 10.1021/acs.jmedchem.5b01020. Epub 2015 Nov 24. J Med Chem. 2016. PMID: 26565590 Free PMC article. Review. - Role of autophagy in methylmercury-induced neurotoxicity in rat primary astrocytes.
Yuntao F, Chenjia G, Panpan Z, Wenjun Z, Suhua W, Guangwei X, Haifeng S, Jian L, Wanxin P, Yun F, Cai J, Aschner M, Rongzhu L. Yuntao F, et al. Arch Toxicol. 2016 Feb;90(2):333-45. doi: 10.1007/s00204-014-1425-1. Epub 2014 Dec 9. Arch Toxicol. 2016. PMID: 25488884 Free PMC article. - Neuroprotective effects of lithium: implications for the treatment of Alzheimer's disease and related neurodegenerative disorders.
Forlenza OV, De-Paula VJ, Diniz BS. Forlenza OV, et al. ACS Chem Neurosci. 2014 Jun 18;5(6):443-50. doi: 10.1021/cn5000309. Epub 2014 May 6. ACS Chem Neurosci. 2014. PMID: 24766396 Free PMC article. Review. - Clarifying lysosomal storage diseases.
Schultz ML, Tecedor L, Chang M, Davidson BL. Schultz ML, et al. Trends Neurosci. 2011 Aug;34(8):401-10. doi: 10.1016/j.tins.2011.05.006. Epub 2011 Jun 30. Trends Neurosci. 2011. PMID: 21723623 Free PMC article. Review.
References
- Autti T, Raininko R, Vanhanen SL, Santavuori P. MRI of neuronal ceroid lipofuscinosis. I. Cranial MRI of 30 patients with juvenile neuronal ceroid lipofuscinosis. Neuroradiology. 1996;38:476–482. - PubMed
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. - PubMed
- Busceti CL, Biagioni F, Aronica E, Riozzi B, Storto M, Battaglia G, Giorgi FS, Gradini R, Fornai F, Caricasole A. Induction of the Wnt inhibitor, Dickkopf-1, is associated with neurodegeneration related to temporal lobe epilepsy. Epilepsia. 2007;48:694–705. - PubMed
- Cao Y, Espinola JA, Fossale E, Massey AC, Cuervo AM, MacDonald ME, Cotman SL. Autophagy is disrupted in a knock-in mouse model of juvenile neuronal ceroid lipofuscinosis. J. Biol. Chem. 2006;281:20483–20493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources