Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions - PubMed (original) (raw)
Soft substrates promote homogeneous self-renewal of embryonic stem cells via downregulating cell-matrix tractions
Farhan Chowdhury et al. PLoS One. 2010.
Abstract
Maintaining undifferentiated mouse embryonic stem cell (mESC) culture has been a major challenge as mESCs cultured in Leukemia Inhibitory Factor (LIF) conditions exhibit spontaneous differentiation, fluctuating expression of pluripotency genes, and genes of specialized cells. Here we show that, in sharp contrast to the mESCs seeded on the conventional rigid substrates, the mESCs cultured on the soft substrates that match the intrinsic stiffness of the mESCs and in the absence of exogenous LIF for 5 days, surprisingly still generated homogeneous undifferentiated colonies, maintained high levels of Oct3/4, Nanog, and Alkaline Phosphatase (AP) activities, and formed embryoid bodies and teratomas efficiently. A different line of mESCs, cultured on the soft substrates without exogenous LIF, maintained the capacity of generating homogeneous undifferentiated colonies with relatively high levels of Oct3/4 and AP activities, up to at least 15 passages, suggesting that this soft substrate approach applies to long term culture of different mESC lines. mESC colonies on these soft substrates without LIF generated low cell-matrix tractions and low stiffness. Both tractions and stiffness of the colonies increased with substrate stiffness, accompanied by downregulation of Oct3/4 expression. Our findings demonstrate that mESC self-renewal and pluripotency can be maintained homogeneously on soft substrates via the biophysical mechanism of facilitating generation of low cell-matrix tractions.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
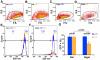
Figure 1. Soft substrates promote mouse embryonic stem cell (mESC) self-renewal.
(A) mESCs on the substrates of 0.6 kPa stiffness [Gel (0.6 kPa)] always formed round and compact colonies (left) with uniform Oct3/4::GFP expression (middle) and the high AP activity (right) in the presence of LIF (LIF+). Arrowheads indicate that marked colonies were washed out during the staining procedure to measure the AP activity. (B) mESCs on the rigid substrates of polystyrene dishes (Rigid dish) with LIF formed round colonies and a spread irregular colony (left; white arrows) with heterogeneous Oct3/4::GFP expression (middle) and varying degrees of the AP activity (right). (C) mESCs on the soft substrates without LIF for 3 days (LIF− 3 days) still formed round colonies with uniform Oct3/4::GFP expression and the AP activity maintained. (D) mESCs on the rigid dish without LIF for 3 days exhibited irregular spread colonies with _Oct3/4::_GFP expression and the AP activity reduced dramatically. (E) The soft substrates supported mESC self-renewal without LIF for 5 days (LIF− 5days) with high uniform Oct3/4::GFP expression and the AP activity maintained. (F) On the rigid dishes, 5 days of culture without LIF resulted in irregular spread colonies with extremely low Oct3/4::GFP expression and a undetectable AP activity.(G–H) Immunocytochemistry with mESCs maintained on the soft (G) or the rigid substrates (H) without LIF for 5 days. Images for bright field (left) and nuclear staining with DAPI (middle) show appearance of colonies. High Nanog expression was observed in the mESCs on the soft substrates (G, right), but not in the ones on the rigid dish (H, right). Three independent experiments showed very similar results. Bars, 100 (A–F) or 50 (G & H) µms.
Figure 2. Quantification of Oct3/4 expression mESCs on soft substrates or rigid substrates.
(A–D) Representative density plots for FACS (fluorescence-activated cell sorting) of mESCs in each condition are shown. The x-axis is for forward scatter and the y-axis, side scatter. An identical gate was applied to all conditions. LIF− condition on rigid dishes yields less number of cells as some cells lose adhesion and float away . This can be seen in the density plot in (D). (E) Representative plots showing high Oct3/4::GFP expression (530 nm) found in cells maintained in the presence (blue) or absence (orange) of LIF. The threshold of GFP expression is arbitrarily determined according to the result from sorting wild-type mESCs (W4) that do not express any fluorescent protein. Two or three percentages of sorted mESCs on the soft substrates with or without LIF are GFP-negative, respectively. (F) The percentage of GFP-negative mESCs increased to 21% of sorted mESCs on the rigid substrates without LIF from 3.2% of those with LIF. (G) Data summary shows Oct3/4::GFP-positive mESCs on the soft substrates or the rigid substrates with or without LIF. An identical gate was applied to all replicates. Mean ± s.e. (n = 4); at least three independent experiments.
Figure 3. Functional validation and transcript analysis of mESCs on soft substrates.
(A) Efficiencies of embryoid body (EB) formation are compared among of mESCs cultured on soft substrates and rigid dishes with or without LIF. mESCs on soft substrate retain higher EB forming capacity even in the absence of LIF as compared to those on rigid dishes. (B) Semi-quantitative RT-PCR was carried out with cDNAs from mESCs cultured in LIF+ and LIF− medium for 5 days either on the soft substrates (G) or the rigid substrates (R). Expression of pluripotency markers Oct3/4, Esg1, Sox2 and Tcf15, the pan-mesodermal maker Brachyury (T), the late mesodermal maker Twist2, and the tumorigenic marker Eras were analyzed. Ef1α is a loading control. Duplicates showed similar results. (C) mESCs cultured on soft substrates without LIF for 5 days developed a teratoma, when injected into NOD-SCID mice subcutaneously, giving rise to all three germ layers. Ne, neural tissue; Ca, cartilage; Mu, Mucous membrane; Ep, epidermis; Ke, keratin pearl; Cc, chondroitin sulfate-rich cartilage; Ci, ciliated epithelium. Bars, 50 µm. (D) mESCs cultured on soft substrates with LIF for 5 days developed a teratoma. The paraffin-embedded teratoma sections confirmed the presence of all three germ layers by immunostaining (nestin: ectoderm, α-fetoprotein: endoderm, and α-smooth muscle actin: mesoderm). Bars, 20 and 50 µm as indicated.
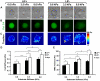
Figure 4. Elevated endogenous stress and stiffness lead to mESC differentiation.
(A) Bright-field images of colonies on 0.6, 3.5 or 8 kPa substrates with or without LIF. Colonies are round and compact on 0.6 kPa substrates in the presence and absence of LIF. In contrast, colonies on 3.5 kPa, similar to 8 kPa substrates, are spread in the presence of LIF and even more spread and irregular in the absence of LIF. (B) Corresponding GFP images of Oct3/4 expression of the same colonies on 0.6, 3.5 or 8 kPa substrates. Uniform Oct3/4::GFP expression is found in colonies on 0.6 kPa substrates but not on 3.5 and 8 kPa substrates. (C) Colonies on 0.6 kPa substrates exert lower tractions than colonies on 3.5 and 8 kPa substrates. (D) Summarized data shows that stiffnesses of the colonies are significantly different between 0.6 and 3.5 kPa substrates, and between 0.6 and 8 kPa substrates, but similar between 3.5 and 8 kPa (all are in LIF+ conditions). Colony stiffnesses are similar with (n = 52) or without (n = 50) LIF on 0.6 kPa substrates, but are significantly different between with (n = 22) or without (n = 19) LIF on 3.5 kPa, and on 8 kPa substrates (n = 85, 10 colonies with or without LIF). Mean ± s.e. (E) RMS (root-mean-square) tractions of colonies on 0.6, 3.5 or 8 kPa substrates. In the presence of LIF, when substrate stiffness increased from 0.6 kPa to 3.5 kPa or to 8 kPa, tractions significantly increased. Tractions on 0.6 kPa were similar with (n = 8) or without (n = 7) LIF; tractions on 3.5 kPa were also similar with (n = 7) or without (n = 6) LIF, but tractions on 8 kPa substrates were different with (n = 6) or without (n = 7) LIF. Mean ± s.e. Bars, 50 µm. (*, p<0.05; **, p<0.01; ***, p<0.001; #, p>0.05)
Similar articles
- Gene expression profiling reveals the heterogeneous transcriptional activity of Oct3/4 and its possible interaction with Gli2 in mouse embryonic stem cells.
Li Y, Drnevich J, Akraiko T, Band M, Li D, Wang F, Matoba R, Tanaka TS. Li Y, et al. Genomics. 2013 Nov-Dec;102(5-6):456-67. doi: 10.1016/j.ygeno.2013.09.004. Epub 2013 Oct 8. Genomics. 2013. PMID: 24121003 - Maintenance of murine embryonic stem cells' self-renewal and pluripotency with increase in proliferation rate by a bovine granulosa cell line-conditioned medium.
Losino N, Luzzani C, Solari C, Boffi J, Tisserand ML, Sevlever G, Barañao L, Guberman A. Losino N, et al. Stem Cells Dev. 2011 Aug;20(8):1439-49. doi: 10.1089/scd.2010.0336. Epub 2011 Jan 12. Stem Cells Dev. 2011. PMID: 21126164 - Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor.
Hirai H, Karian P, Kikyo N. Hirai H, et al. Biochem J. 2011 Aug 15;438(1):11-23. doi: 10.1042/BJ20102152. Biochem J. 2011. PMID: 21793804 Free PMC article. Review. - Animal embryonic stem (ES) cells: self-renewal, pluripotency, transgenesis and nuclear transfer.
Saito S, Liu B, Yokoyama K. Saito S, et al. Hum Cell. 2004 Sep;17(3):107-15. doi: 10.1111/j.1749-0774.2004.tb00026.x. Hum Cell. 2004. PMID: 15859155 Review.
Cited by
- Biophysical regulation of stem cell behavior within the niche.
Conway A, Schaffer DV. Conway A, et al. Stem Cell Res Ther. 2012 Dec 14;3(6):50. doi: 10.1186/scrt141. Stem Cell Res Ther. 2012. PMID: 23241436 Free PMC article. Review. - COL2A1 Is a Novel Biomarker of Melanoma Tumor Repopulating Cells.
Talluri B, Amar K, Saul M, Shireen T, Konjufca V, Ma J, Ha T, Chowdhury F. Talluri B, et al. Biomedicines. 2020 Sep 18;8(9):360. doi: 10.3390/biomedicines8090360. Biomedicines. 2020. PMID: 32962144 Free PMC article. - Generation of organized germ layers from a single mouse embryonic stem cell.
Poh YC, Chen J, Hong Y, Yi H, Zhang S, Chen J, Wu DC, Wang L, Jia Q, Singh R, Yao W, Tan Y, Tajik A, Tanaka TS, Wang N. Poh YC, et al. Nat Commun. 2014 May 30;5:4000. doi: 10.1038/ncomms5000. Nat Commun. 2014. PMID: 24873804 Free PMC article. - A Cross-Linked Cyclosiloxane Polymer Matrix as a Platform Enabling Long-Term Culture of Human Induced Pluripotent Stem Cells with Naïve-Like Features.
Seo C, Song J, Choi Y, Kim T, Lee D, Jon S. Seo C, et al. Biomater Res. 2025 Apr 28;29:0197. doi: 10.34133/bmr.0197. eCollection 2025. Biomater Res. 2025. PMID: 40296880 Free PMC article. - The first embryo, the origin of cancer and animal phylogeny. V. Cancer stem cells as the unifying biomechanical principle between embryology and oncology.
Cofre J. Cofre J. Mechanobiol Med. 2024 Dec 5;3(1):100110. doi: 10.1016/j.mbm.2024.100110. eCollection 2025 Mar. Mechanobiol Med. 2024. PMID: 40396136 Free PMC article. Review.
References
- Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, et al. Nanog safeguards pluripotency and mediates germline development. Nature. 2007;450:1230–1234. - PubMed
- Singh AM, Hamazaki T, Hankowski KE, Terada N. A heterogeneous expression pattern for Nanog in embryonic stem cells. Stem Cells. 2007;25:2534–2542. - PubMed
- Furusawa T, Ikeda M, Inoue F, Ohkoshi K, Hamano T, et al. Gene expression profiling of mouse embryonic stem cell subpopulations. Biol Reprod. 2006;75:555–561. - PubMed
- Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials