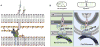Regulation of peptidoglycan synthesis by outer-membrane proteins - PubMed (original) (raw)
. 2010 Dec 23;143(7):1097-109.
doi: 10.1016/j.cell.2010.11.038.
Manuel Banzhaf, Bart van den Berg van Saparoea, Jolanda Verheul, Jacob Biboy, Robert J Nichols, Matylda Zietek, Katrin Beilharz, Kai Kannenberg, Moritz von Rechenberg, Eefjan Breukink, Tanneke den Blaauwen, Carol A Gross, Waldemar Vollmer
Affiliations
- PMID: 21183073
- PMCID: PMC3060616
- DOI: 10.1016/j.cell.2010.11.038
Regulation of peptidoglycan synthesis by outer-membrane proteins
Athanasios Typas et al. Cell. 2010.
Abstract
Growth of the mesh-like peptidoglycan (PG) sacculus located between the bacterial inner and outer membranes (OM) is tightly regulated to ensure cellular integrity, maintain cell shape, and orchestrate division. Cytoskeletal elements direct placement and activity of PG synthases from inside the cell, but precise spatiotemporal control over this process is poorly understood. We demonstrate that PG synthases are also controlled from outside of the sacculus. Two OM lipoproteins, LpoA and LpoB, are essential for the function, respectively, of PBP1A and PBP1B, the major E. coli bifunctional PG synthases. Each Lpo protein binds specifically to its cognate PBP and stimulates its transpeptidase activity, thereby facilitating attachment of new PG to the sacculus. LpoB shows partial septal localization, and our data suggest that the LpoB-PBP1B complex contributes to OM constriction during cell division. LpoA/LpoB and their PBP-docking regions are restricted to γ-proteobacteria, providing models for niche-specific regulation of sacculus growth.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1
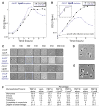
Identification of two OM lipoproteins that regulate the activity of the major_E. coli_ PG synthases. A. The growth phenotypes of _lpoB_−(ycfM) and _mrcB_−cluster strongly across 324 different conditions (cc = 0.9; p<10−116). Cellular fitness is depicted using a color scale: red (increased); green (decreased) fitness. The upper panel illustrates that the highly correlated growth phenotypes of the two mutant strains depend on strong responses to only a few of the 324 conditions tested; the lower panel (blow-up) shows that these conditions are sub-lethal doses of β-lactams (target TPase domain of PBPs) and A22 (targets MreB). B–C. _lpoB_− is synthetically lethal with both_mrcA_− and_lpoA_−. Using high-throughput Hfr mating, we produced a 12 × 12 genetic interaction matrix. Results from pseudo-Hfr lpoB::cat crossed with 12 KanRrecipients arrayed in 1536 format (boxes of 4 × 32 = 128 replicas) on LB are shown in (B) and quantified in (C). Recipients are indicated above the double mutant plate (B) and have colony sizes similar to the wildtype as single mutants (data not shown); the self mating control (lpoB::cat x lpoB::kan; red), demonstrates the low false-positive rate, since a double mutant of the same gene cannot be made in haploid organisms; the white box is a sterility control. _lpoB_− is synthetically lethal with _mrcA_− and_lpoA_−, and synthetically sick with deletions of all _tol_-pal components. The other 6 genetic interactions are neutral. Error bars depict standard deviations (n = 128). _lpoA_− is synthetically lethal with both _mrcB_− and_lpoB_− (Fig. S1A–B).D–E. _lpoB_− and_lpoA_− show epistatic genetic interactions with _mrcB_− (D) and _mrcA_− (E) respectively. Quantifications of growth of wildtype, single mutant and the double mutant strains arrayed in 384-format (n = 96 colonies each) on LB agar plates containing different antibiotics (from Fig. S1C–F). Double mutant phenotypes are similar to single lpo mutant phenotypes indicating that each Lpo protein is absolutely required for the activity of its cognate PBP. F. Summary of genetic and physical interactions between Lpo proteins and PBP1A–PBP1B.
Figure 2
Each Lpo protein physically interacts with its cognate PG synthase in vitro and in vivo. LpoA specifically interacts with PBP1A (A, C), using its C-terminal domain (E); LpoB specifically interacts with PBP1B (B,D). Affinity chromatography with an E. coli membrane fraction applied to sepharose columns with different immobilized proteins; empty sepharose columns serve as controls. The membrane fraction (M) was applied to the columns in the presence of 400 mM NaCl to detect strong interactions and the flowthrough was collected (F). After washing (W), retained proteins were eluted with buffer containing 2 M NaCl (E). Samples were subjected to SDS-PAGE and Western blotting, followed by immunodetection of Lpo proteins or PBPs. Note that PBP1B has a slight nonspecific binding to the sepharose column (A). Lpo proteins also localize to the OM and interact with PG (Fig. S2).F–G. LpoA and LpoB interact with their cognate PBP_in vivo. In vivo_ cross-linking of Lpo proteins with PBPs. E. coli cells were treated with DTSSP cross-linker, and membrane fractions were isolated and immunoprecipitated either with LpoA or PBP1B antibodies (+) or without antibodies (-). Samples were incubated with protein G-agarose beads, centrifuged, and the supernatant collected. The beads were washed and resuspended (protein G samples). Supernatant and protein G samples were boiled in buffer with reducing agent to revert the crosslinking, and eluates were subject to SDS-PAGE and Western Blotting, followed by immunodetection of PBP1A or LpoB.
Figure 3
LpoA and LpoB are absolutely required for the _in vivo_function of their cognate PBP and strongly stimulate the TPase activity of their cognate PBP in vitro. **A–B.**Depletion of Lpo proteins in the absence of the non-cognate PBP leads to lysis. LpoA (A) and LpoB (B) were expressed from an arabinose (Ara)-inducible plasmid in_mrcB_− and_mrcA_− cells respectively, and depleted by dilution of stationary phase cultures into glucose-containing LB medium (repression). For LpoB-depletion, diluted cultures were first grown to OD578=0.6 in glucose LB medium (B, blue line, inset) and then rediluted into fresh glucose LB medium to observe lysis. C–E. Morphology of Lpo-depleted cells. Cells grown with glucose to deplete LpoA (in_mrcB_− background) and LpoB (in_mrcA_− background), or with Ara (control), were fixed and examined by phase contrast microscopy. Lysis of LpoA- or LpoB-depleted cells began after 300 min of growth in glucose. Magnified pictures of LpoA- (D) or LpoB-depleted (E) cells at 300 min reveal the presence of lysis bulges often emerging at midcell (arrows). (F) The activity of detergent-solubilized PBP1A or PBP1B was assayed with radiolabelled lipid II in the presence or absence of their cognate Lpo protein. The PG product was digested with cellosyl and the resulting muropeptides were analysed by HPLC (for chromatograms see Fig. S3). The table shows a summary of the types of muropeptides and properties of the PG synthesized. The % peptides in cross-links was calculated as 100% − % Monomers; the degree of cross-linkage is defined as %Dimers/2 + %Trimers × 2/3 + %Tetramers × 3/4 and is equal to the percent peptides that were used as donors in TPase reactions; n.d., not detected. Both Lpo proteins increased the cross-linkage in the PG synthesized by their cognate PBP. LpoA also stimulated the PBP1A-catalysed attachment of newly made PG to sacculi (Fig. S4).
Figure 4
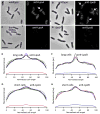
LpoA and LpoB localize as distinct foci in the lateral wall and at constriction sites of dividing cells. E. coli wildtype (TB28) (A) and its _lpoA_−derivative (C) were immunolabeled with antibodies against LpoA.E. coli wildtype (BW25113) (B) and its_lpoB_− derivative (D) were immunolabeled with affinity-purified antibodies against LpoB. The immunolocalization procedure does not affect the cell membrane (Fig. S5A) or the size/shape of the cells (Supplemental Experimental Procedures). The left side of each dual panel shows the phase contrast image and the right side the corresponding fluorescence image. The scale bar equals 5 μm. Arrows in panels A & B depict LpoA and LpoB foci for cells engaged in septation. Panels E–H show the average LpoA (E & G) or LpoB (F &H) fluorescence intensity profiles of >1000 individual cells per strain plotted against the relative position along the length axis of the cell. The populations of cells were split into longer cells (1/3 of the population), enriched in dividing cells (E &F) and shorter cells (2/3 of the population), including only few dividing cells (G & H). For panelsE–H: black lines: wildtype cells; red lines:_lpoA_− cells; blue lines:_lpoB_− cells; green lines:_mrcA_− cells (lacking PBP1A) and purple lines: _mrcB_− cells (lacking PBP1B). The grey line in panels E & G are from a general membrane staining using BODIPY 558/568 C12. LpoB localizes late in the cell cycle to midcell (Fig. S5B). Midcell localization of LpoB depends on the presence of FtsZ, PBP3 and ongoing septal PG synthesis (Fig. S6).
Figure 5
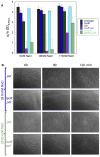
LpoB-PBP1B has a secondary role in OM invagination during cell division.A. OD578 of various strains measured after overnight growth (o/n) in LB with different amounts of salt._lpoB_IM indicates an IM-localized variant of LpoB, created by changing its lipoprotein sorting signal. Lysis phenotypes of_lpoB_−_pal_−and_lpoB_IM_pal_−cells are indistinguishable and are synthetic when compared to the lysis patterns of the individual single mutants. Error bars are based on n>6 repetitions of the growth experiments. The large error bars for_lpoB_−_pal_−and_lpoB_IM_pal_−are likely due to suppressors arising at different time points during the slow growth and continuous lysis of these mutants at low salt concentrations, as all biological repetitions exhibited significant cellular debris, independent of the overnight OD578. Fig. S7 demonstrates that LpoBIM was still able to partially activate PBP1B as it sustained viability in cells lacking either PBP1A or LpoA in LB no/low salt.B. Cellular morphologies of_pal_− and_lpoB_−_pal_−cells in LB containing no or low salt. Cells grown overnight in LB Miller (170 mM NaCl) were inoculated in LB containing no or low salt to an OD of 0.02, and then fixed and examined by phase contrast microscopy at regular intervals thereafter.
Figure 6
LpoA/LpoB and their docking domains in PBP1A/PBP1B have recently evolved together. A. Schematic representation of PBP1A and PBP1B, illustrating the conserved TPase and TGase domains of both proteins, as well as the newly evolved UB2H domain in PBP1B and the comparably sized insertion region ODD in PBP1A. B. Phylogenetic distribution of Lpo proteins and PBP1A/PBP1B with or without the docking regions. STRING (Jensen et al., 2009) was used for assessing protein and domain conservation over >400 bacterial species. ODD and LpoA are limited to γ-proteobacteria (red and yellow lines) and UB2H and LpoB are further restricted to enterobacteria (yellow lines); stringent cutoffs were used to assess conservation of LpoA and LpoB (100 bits), and of UB2H and ODD domains within the class A PBPs (35% amino acid sequence identity). Note that exceptions exist for some large bacterial clades depicted here; for example in the Firmicutes phylum, Mycoplasmae and Ureoplasma have no class A PBP, whereas staphylococci have only one class A PBP that has similar levels of homology to PBP1A and PBP1B. **C.**UB2H is the PBP1B docking domain of LpoB. LpoB does not interact with a PBP1B variant that lacks the UB2H (PBP1BΔUB2H). In vivo cross-linking/co-immunoprecipitation of LpoB with anti-PBP1B was performed as in Fig. 2G.D. ODD is the PBP1A docking region of LpoA. Overexpression of ODD with an N-terminal signal sequence for periplasmic localization (pssODD) leads to lysis in cells that depend on a functional PBP1A-LpoA complex [_mrcB_− (green diamonds) and _lpoB_− (blue circles)], but does not affect wildtype cells (black squares). Note that the OD axis is in log10 and there is a ~25% drop in cell culture density for _mrcB_− and_lpoB_− cells, leading to clear formation of cellular debris. Overexpression of LpoA together with pssODD averts lysis (inset panel).
Figure 7
Model for the mechanism of action of Lpo proteins. A. The docking domain of the PBP interacts with its cognate Lpo, and undergoes a conformational change that repositions its TPase domain so that peptide crosslinking is stimulated. Glycan chains are sandwiched between the IM and OM, and are composed of N-acetylglucosamine (G) and N-acetylmuramic acid (M) depicted as hexagons. Attached to the M sugar are short peptides (balls) that crosslink the glycan strands. The 3-domain PBP is anchored to the IM [blue:TPase; green:GTase; orange: docking domain (UB2H/ODD)], and the Lpo protein (cylinder) is anchored to the OM.B. PBP1A-LpoA & PBP1B-LpoB are primarily responsible respectively for sidewall and septal PG synthesis. Cytoskeletal elements and the large elongasome/divisome complexes assembled around them recruit PBP1A at the lateral wall of elongating cells and PBP1B at septa of dividing cells. Here, IM components of these complexes are depicted as colored ovals, and periplasmic/OM components, including PG hydrolases and other PBPs, are omitted for clarity. LpoA and LpoB mirror the localization of their cognate PBP. Lpo proteins localize independently of their cognate PBP possibly via interaction with newly synthesized PG and/or via yet unidentified interactions to elongasome/divisome members. Despite their localization preferences, each PBP-Lpo complex can substitute for the loss of the other, which is reflected by the presence of both as foci at the lateral wall of cells and also at midcell of dividing cells. The docking domains for PBP1A (ODD) and PB1B (UB2H) are depicted here in orange and gold respectively.
Comment in
- New ways to make old walls: bacterial surprises.
Young KD. Young KD. Cell. 2010 Dec 23;143(7):1042-4. doi: 10.1016/j.cell.2010.12.011. Cell. 2010. PMID: 21183069
Similar articles
- Outer-membrane lipoprotein LpoB spans the periplasm to stimulate the peptidoglycan synthase PBP1B.
Egan AJ, Jean NL, Koumoutsi A, Bougault CM, Biboy J, Sassine J, Solovyova AS, Breukink E, Typas A, Vollmer W, Simorre JP. Egan AJ, et al. Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8197-202. doi: 10.1073/pnas.1400376111. Epub 2014 May 12. Proc Natl Acad Sci U S A. 2014. PMID: 24821816 Free PMC article. - The LpoA activator is required to stimulate the peptidoglycan polymerase activity of its cognate cell wall synthase PBP1a.
Sardis MF, Bohrhunter JL, Greene NG, Bernhardt TG. Sardis MF, et al. Proc Natl Acad Sci U S A. 2021 Aug 31;118(35):e2108894118. doi: 10.1073/pnas.2108894118. Proc Natl Acad Sci U S A. 2021. PMID: 34429361 Free PMC article. - Lipoprotein cofactors located in the outer membrane activate bacterial cell wall polymerases.
Paradis-Bleau C, Markovski M, Uehara T, Lupoli TJ, Walker S, Kahne DE, Bernhardt TG. Paradis-Bleau C, et al. Cell. 2010 Dec 23;143(7):1110-20. doi: 10.1016/j.cell.2010.11.037. Cell. 2010. PMID: 21183074 Free PMC article. - Activities and regulation of peptidoglycan synthases.
Egan AJ, Biboy J, van't Veer I, Breukink E, Vollmer W. Egan AJ, et al. Philos Trans R Soc Lond B Biol Sci. 2015 Oct 5;370(1679):20150031. doi: 10.1098/rstb.2015.0031. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26370943 Free PMC article. Review. - From the regulation of peptidoglycan synthesis to bacterial growth and morphology.
Typas A, Banzhaf M, Gross CA, Vollmer W. Typas A, et al. Nat Rev Microbiol. 2011 Dec 28;10(2):123-36. doi: 10.1038/nrmicro2677. Nat Rev Microbiol. 2011. PMID: 22203377 Free PMC article. Review.
Cited by
- Multi-copy genes that enhance the yield of mammalian G protein-coupled receptors in Escherichia coli.
Skretas G, Makino T, Varadarajan N, Pogson M, Georgiou G. Skretas G, et al. Metab Eng. 2012 Sep;14(5):591-602. doi: 10.1016/j.ymben.2012.05.001. Epub 2012 May 17. Metab Eng. 2012. PMID: 22609824 Free PMC article. - Small-molecule inhibitors of gram-negative lipoprotein trafficking discovered by phenotypic screening.
McLeod SM, Fleming PR, MacCormack K, McLaughlin RE, Whiteaker JD, Narita S, Mori M, Tokuda H, Miller AA. McLeod SM, et al. J Bacteriol. 2015 Mar;197(6):1075-82. doi: 10.1128/JB.02352-14. Epub 2015 Jan 12. J Bacteriol. 2015. PMID: 25583975 Free PMC article. - Upregulation of PBP1B and LpoB in cysB Mutants Confers Mecillinam (Amdinocillin) Resistance in Escherichia coli.
Thulin E, Andersson DI. Thulin E, et al. Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00612-19. doi: 10.1128/AAC.00612-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31332059 Free PMC article. - Proteome and Physiological Characterization of Halotolerant Nodule Endophytes: The Case of Rahnella aquatilis and Serratia plymuthica.
Novello G, Gamalero E, Massa N, Cesaro P, Lingua G, Todeschini V, Caramaschi A, Favero F, Corà D, Manfredi M, Marengo E, Pelagi M, Pangaro L, Caffiero G, Milano F, Bona E. Novello G, et al. Microorganisms. 2022 Apr 24;10(5):890. doi: 10.3390/microorganisms10050890. Microorganisms. 2022. PMID: 35630335 Free PMC article. - The Pathogenic Neisseria Use a Streamlined Set of Peptidoglycan Degradation Proteins for Peptidoglycan Remodeling, Recycling, and Toxic Fragment Release.
Schaub RE, Dillard JP. Schaub RE, et al. Front Microbiol. 2019 Jan 31;10:73. doi: 10.3389/fmicb.2019.00073. eCollection 2019. Front Microbiol. 2019. PMID: 30766523 Free PMC article. Review.
References
- Aaron M, Charbon G, Lam H, Schwarz H, Vollmer W, Jacobs-Wagner C. The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol. 2007;64:938–952. - PubMed
- Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Distèche M, den Blaauwen T. Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol. 2005;55:1631–1645. - PubMed
- Adams DW, Errington J. Bacterial cell division: assembly, maintenance and disassembly of the Z ring. Nat Rev Microbiol. 2009;7:642–653. - PubMed
- Bertsche U, Breukink E, Kast T, Vollmer W. In vitro murein peptidoglycan synthesis by dimers of the bifunctional transglycosylase-transpeptidase PBP1B from Escherichia coli. J Biol Chem. 2005;280:38096–38101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM085697/GM/NIGMS NIH HHS/United States
- BB/F001231/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- K99 GM092984/GM/NIGMS NIH HHS/United States
- F31 DE020206-01/DE/NIDCR NIH HHS/United States
- R01 GM036278/GM/NIGMS NIH HHS/United States
- F31 DE020206/DE/NIDCR NIH HHS/United States
- K99GM092984/GM/NIGMS NIH HHS/United States
- T32 DE007306/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous